Discontinuing adalimumab in patients with controlled juvenile idiopathic arthritis-associated uveitis (ADJUST-Adalimumab in Juvenile Idiopathic Arthritis-associated Uveitis Stopping Trial): study protocol for a randomised controlled trial
- PMID: 33109240
- PMCID: PMC7590716
- DOI: 10.1186/s13063-020-04796-z
Discontinuing adalimumab in patients with controlled juvenile idiopathic arthritis-associated uveitis (ADJUST-Adalimumab in Juvenile Idiopathic Arthritis-associated Uveitis Stopping Trial): study protocol for a randomised controlled trial
Abstract
Background: Juvenile idiopathic arthritis (JIA)-associated uveitis is a chronic paediatric ocular inflammatory condition that can result in visual impairment. Adalimumab, a tumour necrosis factor (TNF)-alpha inhibitor, effectively controls joint and eye inflammation; however, its long-term use may increase the risk of adverse health outcomes and place an undue financial burden on the patient and healthcare system given its high cost. There is great interest for patients to stop adalimumab following remission due to these reasons but there is a lack of information on the ability to maintain control after discontinuing adalimumab.
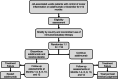
Methods: The Adalimumab in Juvenile Idiopathic Arthritis-associated Uveitis Trial (ADJUST) is a multicentred, international trial that will randomise 118 participants aged 2 years and older with controlled JIA-associated uveitis to either continue adalimumab or discontinue adalimumab and receive a placebo. The trial will compare the time to uveitis recurrence between the two groups over 12 months. All participants will receive the standard weight-based dose of adalimumab or placebo: 20 mg biweekly (if < 30 kg) or 40 mg biweekly (if ≥ 30 kg).
Discussion: This is the first randomised controlled trial to assess the efficacy of discontinuing adalimumab after demonstrating control of JIA-associated uveitis for at least 12 months. The results of ADJUST will provide information on clinical outcomes to guide clinicians in their decision-making regarding discontinuation of adalimumab.
Trial registration: ClinicalTrials.gov NCT03816397. Registered on 25 January 2019. EudraCT 2019-000412-29. Registered on 17 January 2019.
Keywords: Adalimumab; Juvenile idiopathic arthritis; Randomised controlled trial; Uveitis.
Figures
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical