Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3
- PMID: 33109253
- PMCID: PMC7590698
- DOI: 10.1186/s13075-020-02325-6
Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3
Abstract
Background: Emerging evidence suggests that microRNAs (miRs) are associated with the progression of osteoarthritis (OA). In this study, the role of exosomal miR-136-5p derived from mesenchymal stem cells (MSCs) in OA progression is investigated and the potential therapeutic mechanism explored.
Methods: Bone marrow mesenchymal stem cells (BMMSCs) and their exosomes were isolated from patients and identified. The endocytosis of chondrocytes and the effects of exosome miR-136-5p on cartilage degradation were observed and examined by immunofluorescence and cartilage staining. Then, the targeting relationship between miR-136-5p and E74-like factor 3 (ELF3) was analyzed by dual-luciferase report assay. Based on gain- or loss-of-function experiments, the effects of exosomes and exosomal miR-136-5p on chondrocyte migration were examined by EdU and Transwell assay. Finally, a mouse model of post-traumatic OA was developed to evaluate effects of miR-136-5p on chondrocyte degeneration in vivo.
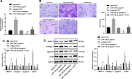
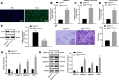
Results: In the clinical samples of traumatic OA cartilage tissues, we detected increased ELF3 expression, and reduced miR-136-5p expression was determined. The BMMSC-derived exosomes showed an enriched level of miR-136-5p, which could be internalized by chondrocytes. The migration of chondrocyte was promoted by miR-136-5p, while collagen II, aggrecan, and SOX9 expression was increased and MMP-13 expression was reduced. miR-136-5p was verified to target ELF3 and could downregulate its expression. Moreover, the expression of ELF3 was reduced in chondrocytes after internalization of exosomes. In the mouse model of post-traumatic OA, exosomal miR-136-5p was found to reduce the degeneration of cartilage extracellular matrix.
Conclusion: These data provide evidence that BMMSC-derived exosomal miR-136-5p could promote chondrocyte migration in vitro and inhibit cartilage degeneration in vivo, thereby inhibiting OA pathology, which highlighted the transfer of exosomal miR-136-5p as a promising therapeutic strategy for patients with OA.
Keywords: Bone marrow mesenchymal stem cells; Chondrocyte degeneration; ELF3; Exosome; Extracellular matrix secretion; Migration; Traumatic osteoarthritis; microRNA-136-5p.
Conflict of interest statement
None
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

