Considering complexity in the genetic evaluation of dilated cardiomyopathy
- PMID: 33109712
- PMCID: PMC8283773
- DOI: 10.1136/heartjnl-2020-316658
Considering complexity in the genetic evaluation of dilated cardiomyopathy
Abstract
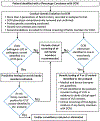
Dilated cardiomyopathy (DCM) is a cardiovascular disease of genetic aetiology that causes substantial morbidity and mortality, and presents considerable opportunity for disease mitigation and prevention in those at risk. Foundational to the process of caring for patients diagnosed with DCM is a clinical genetic evaluation, which always begins with a comprehensive family history and clinical evaluation. Genetic testing of the proband, the first patient identified in a family with DCM, within the context of genetic counselling is always indicated, regardless of whether the DCM is familial or non-familial. Clinical screening of at-risk family members is also indicated, as is cascade genetic testing for actionable variants found at genetic testing in the proband. Clinicians now have expansive panels with many genes available for DCM genetic testing, and the approaches used to evaluate rare variants to decide which are disease-causing continues to rapidly evolve. Despite these recent advances, only a minority of cases yield actionable variants, even in familial DCM where a genetic aetiology is highly likely. This underscores that our knowledge of DCM clinical genetics remains incomplete, including variant interpretation and DCM genetic architecture. Emerging data suggest that the single-variant Mendelian disease model is insufficient to explain some DCM cases, and rather that multiple variants, both common and rare, and at times key environmental factors, interact to cause DCM. A simple model illustrating the intersection of DCM genetic architecture with environmental impact is provided.
Keywords: genetics; idiopathic dilated cardiomyopathy.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals.Genet Med. 2010 Nov;12(11):655-67. doi: 10.1097/GIM.0b013e3181f2481f. Genet Med. 2010. PMID: 20864896 Free PMC article.
-
Evidence-Based Assessment of Genes in Dilated Cardiomyopathy.Circulation. 2021 Jul 6;144(1):7-19. doi: 10.1161/CIRCULATIONAHA.120.053033. Epub 2021 May 5. Circulation. 2021. PMID: 33947203 Free PMC article.
-
Familial Dilated Cardiomyopathy.Heart Lung Circ. 2020 Apr;29(4):566-574. doi: 10.1016/j.hlc.2019.11.018. Epub 2019 Dec 17. Heart Lung Circ. 2020. PMID: 31974027 Review.
-
Retrospective Analysis of Clinical Genetic Testing in Pediatric Primary Dilated Cardiomyopathy: Testing Outcomes and the Effects of Variant Reclassification.J Am Heart Assoc. 2020 Jun 2;9(11):e016195. doi: 10.1161/JAHA.120.016195. Epub 2020 May 27. J Am Heart Assoc. 2020. PMID: 32458740 Free PMC article.
-
Genetics of dilated cardiomyopathy.Heart Fail Clin. 2010 Apr;6(2):129-40. doi: 10.1016/j.hfc.2009.11.003. Heart Fail Clin. 2010. PMID: 20347783 Review.
Cited by
-
Challenges and Applications of Genetic Testing in Dilated Cardiomyopathy: Genotype, Phenotype and Clinical Implications.Arq Bras Cardiol. 2023 Nov;120(10):e20230174. doi: 10.36660/abc.20230174. Arq Bras Cardiol. 2023. PMID: 38055534 Free PMC article. English, Portuguese.
-
Evolving cardiovascular genetic counseling needs in the era of precision medicine.Front Cardiovasc Med. 2023 Jun 23;10:1161029. doi: 10.3389/fcvm.2023.1161029. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37424912 Free PMC article.
-
Human Genetics of Cardiomyopathies.Adv Exp Med Biol. 2024;1441:977-990. doi: 10.1007/978-3-031-44087-8_63. Adv Exp Med Biol. 2024. PMID: 38884765
-
The DCM Project Portal: A direct-to-participant platform of The DCM Research Project.medRxiv [Preprint]. 2023 Jun 29:2023.06.22.23291764. doi: 10.1101/2023.06.22.23291764. medRxiv. 2023. Update in: Am Heart J Plus. 2024 Feb;38:100356. doi: 10.1016/j.ahjo.2023.100356. PMID: 37425710 Free PMC article. Updated. Preprint.
-
European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases.J Arrhythm. 2022 May 31;38(4):491-553. doi: 10.1002/joa3.12717. eCollection 2022 Aug. J Arrhythm. 2022. PMID: 35936045 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention. National Center for Health Statistics: Leading Causes of Death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Published 2017. Accessed 6/22/2020, 2020.
-
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013;10:531–547. - PubMed
-
- Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 2005;45:969–981. - PubMed
-
- Haas J, Frese KS, Peil B, et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J 2015;36:1123–1135a. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
