Time cells in the human hippocampus and entorhinal cortex support episodic memory
- PMID: 33109718
- PMCID: PMC7668099
- DOI: 10.1073/pnas.2013250117
Time cells in the human hippocampus and entorhinal cortex support episodic memory
Abstract
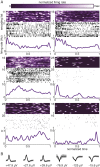
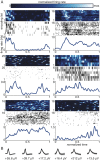
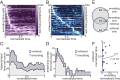
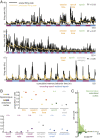
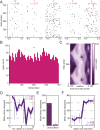
The organization of temporal information is critical for the encoding and retrieval of episodic memories. In the rodent hippocampus and entorhinal cortex, evidence accumulated over the last decade suggests that populations of "time cells" in the hippocampus encode temporal information. We identify time cells in humans using intracranial microelectrode recordings obtained from 27 human epilepsy patients who performed an episodic memory task. We show that time cell activity predicts the temporal organization of retrieved memory items. We also uncover evidence of ramping cell activity in humans, which represents a complementary type of temporal information. These findings establish a cellular mechanism for the representation of temporal information in the human brain needed to form episodic memories.
Keywords: human electrophysiology; medial temporal lobe; theta precession; time cells.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

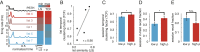
Similar articles
-
Electrical Stimulation in Hippocampus and Entorhinal Cortex Impairs Spatial and Temporal Memory.J Neurosci. 2018 May 9;38(19):4471-4481. doi: 10.1523/JNEUROSCI.3049-17.2018. Epub 2018 Apr 10. J Neurosci. 2018. PMID: 29636396 Free PMC article.
-
Dynamic Theta Networks in the Human Medial Temporal Lobe Support Episodic Memory.Curr Biol. 2019 Apr 1;29(7):1100-1111.e4. doi: 10.1016/j.cub.2019.02.020. Epub 2019 Mar 21. Curr Biol. 2019. PMID: 30905609 Free PMC article.
-
Episodic memory: Neuronal codes for what, where, and when.Hippocampus. 2019 Dec;29(12):1190-1205. doi: 10.1002/hipo.23132. Epub 2019 Jul 23. Hippocampus. 2019. PMID: 31334573 Review.
-
Differential Laminar Activation Dissociates Encoding and Retrieval in the Human Medial and Lateral Entorhinal Cortex.J Neurosci. 2023 Apr 19;43(16):2874-2884. doi: 10.1523/JNEUROSCI.1488-22.2023. Epub 2023 Mar 22. J Neurosci. 2023. PMID: 36948584 Free PMC article.
-
Complementary roles of hippocampus and medial entorhinal cortex in episodic memory.Neural Plast. 2008;2008:258467. doi: 10.1155/2008/258467. Neural Plast. 2008. PMID: 18615199 Free PMC article. Review.
Cited by
-
Phase precession in the human hippocampus and entorhinal cortex.Cell. 2021 Jun 10;184(12):3242-3255.e10. doi: 10.1016/j.cell.2021.04.017. Epub 2021 May 11. Cell. 2021. PMID: 33979655 Free PMC article.
-
Accounting for multiscale processing in adaptive real-world decision-making via the hippocampus.Front Neurosci. 2023 Sep 5;17:1200842. doi: 10.3389/fnins.2023.1200842. eCollection 2023. Front Neurosci. 2023. PMID: 37732307 Free PMC article. Review.
-
Is time an embodied property of concepts?PLoS One. 2023 Sep 5;18(9):e0290997. doi: 10.1371/journal.pone.0290997. eCollection 2023. PLoS One. 2023. PMID: 37669298 Free PMC article.
-
Event Integration and Temporal Differentiation: How Hierarchical Knowledge Emerges in Hippocampal Subfields through Learning.J Neurosci. 2024 Mar 6;44(10):e0627232023. doi: 10.1523/JNEUROSCI.0627-23.2023. J Neurosci. 2024. PMID: 38129134 Free PMC article.
-
What could evolve in the evolution of memory?Philos Trans R Soc Lond B Biol Sci. 2025 Jun 26;380(1929):20240109. doi: 10.1098/rstb.2024.0109. Epub 2025 Jun 26. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40566906 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

