A Small-Molecule Modulator of Metal Homeostasis in Gram-Positive Pathogens
- PMID: 33109764
- PMCID: PMC7593973
- DOI: 10.1128/mBio.02555-20
A Small-Molecule Modulator of Metal Homeostasis in Gram-Positive Pathogens
Abstract
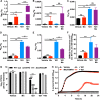
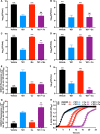
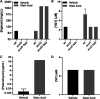
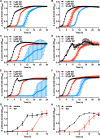
Metals are essential nutrients that all living organisms acquire from their environment. While metals are necessary for life, excess metal uptake can be toxic; therefore, intracellular metal levels are tightly regulated in bacterial cells. Staphylococcus aureus, a Gram-positive bacterium, relies on metal uptake and metabolism to colonize vertebrates. Thus, we hypothesized that an expanded understanding of metal homeostasis in S. aureus will lead to the discovery of pathways that can be targeted with future antimicrobials. We sought to identify small molecules that inhibit S. aureus growth in a metal-dependent manner as a strategy to uncover pathways that maintain metal homeostasis. Here, we demonstrate that VU0026921 kills S. aureus through disruption of metal homeostasis. VU0026921 activity was characterized through cell culture assays, transcriptional sequencing, compound structure-activity relationship, reactive oxygen species (ROS) generation assays, metal binding assays, and metal level analyses. VU0026921 disrupts metal homeostasis in S. aureus, increasing intracellular accumulation of metals and leading to toxicity through mismetalation of enzymes, generation of reactive oxygen species, or disruption of other cellular processes. Antioxidants partially protect S. aureus from VU0026921 killing, emphasizing the role of reactive oxygen species in the mechanism of killing, but VU0026921 also kills S. aureus anaerobically, indicating that the observed toxicity is not solely oxygen dependent. VU0026921 disrupts metal homeostasis in multiple Gram-positive bacteria, leading to increased reactive oxygen species and cell death, demonstrating the broad applicability of these findings. Further, this study validates VU0026921 as a probe to further decipher mechanisms required to maintain metal homeostasis in Gram-positive bacteria.IMPORTANCEStaphylococcus aureus is a leading agent of antibiotic-resistant bacterial infections in the world. S. aureus tightly controls metal homeostasis during infection, and disruption of metal uptake systems impairs staphylococcal virulence. We identified small molecules that interfere with metal handling in S. aureus to develop chemical probes to investigate metallobiology in this organism. Compound VU0026921 was identified as a small molecule that kills S. aureus both aerobically and anaerobically. The activity of VU0026921 is modulated by metal supplementation, is enhanced by genetic inactivation of Mn homeostasis genes, and correlates with increased cellular reactive oxygen species. Treatment with VU0026921 causes accumulation of multiple metals within S. aureus cells and concomitant upregulation of genes involved in metal detoxification. This work defines a small-molecule probe for further defining the role of metal toxicity in S. aureus and validates future antibiotic development targeting metal toxicity pathways.
Keywords: MRSA; Staphylococcus aureus; antibiotics; cobalt; copper; manganese; metalloregulation.
Copyright © 2020 Juttukonda et al.
Figures




References
-
- Silver LL, Bush K. 2016. Antibiotics and antibiotic resistance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- CDC. 2019. Antibiotic resistance threats in the United States. CDC, Atlanta, GA. https://www.cdc.gov/DrugResistance/Biggest-Threats.html.
-
- WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO, Geneva, Switzerland. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-E....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

