Needle-injectable microcomposite cryogel scaffolds with antimicrobial properties
- PMID: 33110210
- PMCID: PMC7591905
- DOI: 10.1038/s41598-020-75196-1
Needle-injectable microcomposite cryogel scaffolds with antimicrobial properties
Abstract
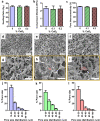
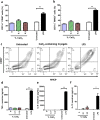
Porous three-dimensional hydrogel scaffolds have an exquisite ability to promote tissue repair. However, because of their high water content and invasive nature during surgical implantation, hydrogels are at an increased risk of bacterial infection. Recently, we have developed elastic biomimetic cryogels, an advanced type of polymeric hydrogel, that are syringe-deliverable through hypodermic needles. These needle-injectable cryogels have unique properties, including large and interconnected pores, mechanical robustness, and shape-memory. Like hydrogels, cryogels are also susceptible to colonization by microbial pathogens. To that end, our minimally invasive cryogels have been engineered to address this challenge. Specifically, we hybridized the cryogels with calcium peroxide microparticles to controllably produce bactericidal hydrogen peroxide. Our novel microcomposite cryogels exhibit antimicrobial properties and inhibit antibiotic-resistant bacteria (MRSA and Pseudomonas aeruginosa), the most common cause of biomaterial implant failure in modern medicine. Moreover, the cryogels showed negligible cytotoxicity toward murine fibroblasts and prevented activation of primary bone marrow-derived dendritic cells ex vivo. Finally, in vivo data suggested tissue integration, biodegradation, and minimal host inflammatory responses when the antimicrobial cryogels, even when purposely contaminated with bacteria, were subcutaneously injected in mice. Collectively, these needle-injectable microcomposite cryogels show great promise for biomedical applications, especially in tissue engineering and regenerative medicine.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials.Gels. 2021 Jun 28;7(3):79. doi: 10.3390/gels7030079. Gels. 2021. PMID: 34203439 Free PMC article. Review.
-
Engineering injectable, biocompatible, and highly elastic bioadhesive cryogels.Mater Today Bio. 2023 Feb 1;19:100572. doi: 10.1016/j.mtbio.2023.100572. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36880083 Free PMC article.
-
Injectable Lignin-co-Gelatin Cryogels with Antioxidant and Antibacterial Properties for Biomedical Applications.Biomacromolecules. 2021 Oct 11;22(10):4110-4121. doi: 10.1021/acs.biomac.1c00575. Epub 2021 Sep 13. Biomacromolecules. 2021. PMID: 34514795
-
Hyaluronic Acid-Based Shape-Memory Cryogel Scaffolds for Focal Cartilage Defect Repair.Tissue Eng Part A. 2021 Jun;27(11-12):748-760. doi: 10.1089/ten.TEA.2020.0264. Epub 2021 Feb 5. Tissue Eng Part A. 2021. PMID: 33108972
-
Injectable Cryogels for Biomedical Applications.Trends Biotechnol. 2020 Apr;38(4):418-431. doi: 10.1016/j.tibtech.2019.09.008. Epub 2019 Nov 5. Trends Biotechnol. 2020. PMID: 31699534 Review.
Cited by
-
Mitigating Metal-Organic Framework (MOF) Toxicity for Biomedical Applications.Chem Eng J. 2023 Sep 1;471:144400. doi: 10.1016/j.cej.2023.144400. Epub 2023 Jun 25. Chem Eng J. 2023. PMID: 39280062 Free PMC article.
-
Injectable Biomimetic Gels for Biomedical Applications.Biomimetics (Basel). 2024 Jul 8;9(7):418. doi: 10.3390/biomimetics9070418. Biomimetics (Basel). 2024. PMID: 39056859 Free PMC article. Review.
-
Effect of lyophilized gelatin-norbornene cryogel size on calvarial bone regeneration.Mater Today Bio. 2023 Nov 27;23:100868. doi: 10.1016/j.mtbio.2023.100868. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38075253 Free PMC article.
-
Designing Silk-Based Cryogels for Biomedical Applications.Biomimetics (Basel). 2022 Dec 22;8(1):5. doi: 10.3390/biomimetics8010005. Biomimetics (Basel). 2022. PMID: 36648791 Free PMC article. Review.
-
Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials.Gels. 2021 Jun 28;7(3):79. doi: 10.3390/gels7030079. Gels. 2021. PMID: 34203439 Free PMC article. Review.
References
-
- O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14:88–95. doi: 10.1016/s1369-7021(11)70058-x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

