Evaluation of cancer-derived myocardial impairments using a mouse model
- PMID: 33110478
- PMCID: PMC7566807
- DOI: 10.18632/oncotarget.27759
Evaluation of cancer-derived myocardial impairments using a mouse model
Abstract
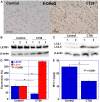
Myocardial damage in cancer patients is emphasized as a cause of death; however, there are not many murine cachexia models to evaluate cancer-derived heart disorder. Using the mouse cachexia model that we established previously, we investigated myocardial damage in tumor-bearing mice. In cachexic mice, decreased heart weight and myocardial volume, and dilated left ventricular lumen, and atrophied cardiomyocytes were noted. The cardiomyocytes also showed accumulated 8-hydroxydeoxyguanosine, decreased leucine zipper and EF-hand-containing transmembrane protein-1, and increased microtubule-associated protein light chain3-II. Levels of tumor necrosis factor-α and high-mobility group box-1 proteins in the myocardium were increased, and nuclear factor κB, a signaling molecule associated with these proteins, was activated. When rat cardiomyoblasts (H9c2 cells) were treated with mouse cachexia model ascites and subjected to flux analysis, both oxidative phosphorylation and glycolysis were suppressed, and the cells were in a quiescent state. These results are in good agreement with those previously reported on cancerous myocardial damage. The established mouse cachexia model can therefore be considered useful for analyzing cancer-derived myocardial damage.
Keywords: atrophy; cachexia; mitochondria; myocardium; oxidative stress.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures




Similar articles
-
Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model.Cancer Sci. 2020 Dec;111(12):4605-4615. doi: 10.1111/cas.14656. Epub 2020 Oct 2. Cancer Sci. 2020. PMID: 32969559 Free PMC article.
-
Regulation of myocardial stromal cell-derived factor 1α/CXCL12 by tumor necrosis factor signaling.J Surg Res. 2017 Jan;207:155-163. doi: 10.1016/j.jss.2016.08.073. Epub 2016 Aug 31. J Surg Res. 2017. PMID: 27979472
-
Astragaloside IV Alleviates the Myocardial Damage Induced by Lipopolysaccharide via the Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-κB)/Proliferator-Activated Receptor α (PPARα) Signaling Pathway.Med Sci Monit. 2019 Sep 23;25:7158-7168. doi: 10.12659/MSM.916030. Med Sci Monit. 2019. PMID: 31545785 Free PMC article.
-
Cancer-induced cardiac cachexia: Pathogenesis and impact of physical activity (Review).Oncol Rep. 2017 May;37(5):2543-2552. doi: 10.3892/or.2017.5542. Epub 2017 Mar 31. Oncol Rep. 2017. PMID: 28393216 Review.
-
The mechanisms of cachexia underlying muscle dysfunction in COPD.J Appl Physiol (1985). 2013 May;114(9):1253-62. doi: 10.1152/japplphysiol.00790.2012. Epub 2012 Sep 27. J Appl Physiol (1985). 2013. PMID: 23019314 Review.
Cited by
-
Left ventricle- and skeletal muscle-derived fibroblasts exhibit a differential inflammatory and metabolic responsiveness to interleukin-6.Front Immunol. 2022 Jul 28;13:947267. doi: 10.3389/fimmu.2022.947267. eCollection 2022. Front Immunol. 2022. PMID: 35967380 Free PMC article.
-
Biomarkers and mechanisms associated with cancer-induced cardiac cachexia: A systematic review.J Cachexia Sarcopenia Muscle. 2023 Aug;14(4):1900-1905. doi: 10.1002/jcsm.13267. Epub 2023 May 21. J Cachexia Sarcopenia Muscle. 2023. PMID: 37211636 Free PMC article. No abstract available.
-
Berberine Improves Cancer-Derived Myocardial Impairment in Experimental Cachexia Models by Targeting High-Mobility Group Box-1.Int J Mol Sci. 2024 Apr 26;25(9):4735. doi: 10.3390/ijms25094735. Int J Mol Sci. 2024. PMID: 38731953 Free PMC article.
-
Cardiac Remodeling in Cancer-Induced Cachexia: Functional, Structural, and Metabolic Contributors.Cells. 2022 Jun 15;11(12):1931. doi: 10.3390/cells11121931. Cells. 2022. PMID: 35741060 Free PMC article. Review.
-
Review of Mechanisms and Treatment of Cancer-Induced Cardiac Cachexia.Cells. 2022 Mar 18;11(6):1040. doi: 10.3390/cells11061040. Cells. 2022. PMID: 35326491 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

