Validation of the DECA criteria for allergic conjunctivitis severity and control
- PMID: 33110491
- PMCID: PMC7585176
- DOI: 10.1186/s13601-020-00349-4
Validation of the DECA criteria for allergic conjunctivitis severity and control
Abstract
Background: Allergic conjunctivitis (AC) is usually associated to allergic rhinitis (AR), but the severity and control of ocular symptoms should be assessed independently to improve diagnosis and treatment. The criteria from the Spanish consensus document on allergic conjunctivitis (DECA) aimed to be used as a patient-reported instrument for AC management. Here we validate these criteria for classifying AC severity and defining its control following COSMIN guidelines recommendations.
Methods: Patients with moderate or severe AR [reflective total nasal symptom score (rTNSS) score ≥ 8] and concomitant AC were recruited from hospitals in Spain. Patients were classified according to the severity of ocular symptoms as mild, moderate, or severe, and classified with respect to control as controlled and non-controlled, using the DECA criteria. To validate these criteria, comparisons with the validated modified allergic rhinitis and its impact on asthma (mARIA), reflective total ocular symptom score (rTOSS), rhinitis control assessment test (RCAT), ESPRINT-15 questionnaires, a conjunctival hyperemia scale and a visual analogue scale (VAS) for ocular symptoms were performed.
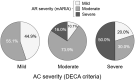
Results: A total of 128 patients participated in the validation. Mean age was 34.4 ± 12.1 years; 72.7% were women. The DECA criteria showed a good discriminant validity, reflecting a high capacity to differentiate between mild, moderate, and severe patients, and controlled from uncontrolled patients. A strong association between AC and AR was reflected in the comparison between the DECA and the mARIA criteria (p < 0.0001). The DECA criteria for severity and control presented satisfactory properties for longitudinal validity and responsiveness.
Conclusions: Validation of the DECA criteria for severity and control of AC suggested that it can be useful in the evaluation of eye symptoms and follow-up of therapies.
Keywords: Allergic conjunctivitis; Allergic rhinoconjunctivitis; Conjunctivitis classification; Control; Ocular allergy.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsMCS-H reports grants from MYLAN during the conduct of the study. Outside this study, she reports personal fees from MYLAN, Sanofi, Novartis, and Merck. AMN reports grants from MYLAN during the conduct of the study. Outside this study, she reports personal fees from GSK, AstraZeneca, Merck, and MYLAN. CC reports having served as a consultant to Mylan and AstraZeneca; having been paid lecture fees by AstraZeneca, GSK, Mylan, and MSD; and grants from Roxall, Novartis, AstraZeneca, and Sanofi. AdC reports grants from MYLAN during the conduct of the study. Outside this study, he reports grants and personal fees from MYLAN, FAES Pharma, Novartis, Allakos, and Sanofi; and personal fees from ALK, GSK, and MSD. JS reports grants from MEDA during the conduct of the study; grants and personal fees from SANOFI; and personal fees from GSK, NOVARTIS, ASTRA ZENECA, MUNDIPHARMA, and FAES FARMA, outside the submitted work. JM reports personal fees and other from SANOFI-GENZYME & REGENERON, NOVARTIS; grants and personal fees from MYLAN-MEDA Pharma and URIACH Group; and personal fees from Mitsubishi-Tanabe, Menarini, UCB, AstraZeneca, GSK, and MSD, outside the submitted work. AV reports grants from MEDA during the conduct of the study.
Figures


References
-
- Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J Allergy Clin Immunol. 2010;126(778–83):e6. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

