Prevention of post-cardiac surgery vitamin D deficiency in children with congenital heart disease: a pilot feasibility dose evaluation randomized controlled trial
- PMID: 33110622
- PMCID: PMC7583219
- DOI: 10.1186/s40814-020-00700-3
Prevention of post-cardiac surgery vitamin D deficiency in children with congenital heart disease: a pilot feasibility dose evaluation randomized controlled trial
Abstract
Background: The vast majority of children undergoing cardiac surgery have low vitamin D levels post-operative, which may contribute to greater illness severity and worse clinical outcomes. Prior to the initiation of a large phase III clinical trial focused on clinical outcomes, studies are required to evaluate the feasibility of the study protocol, including whether the proposed dosing regimen can safely prevent post-operative vitamin D deficiency in this high-risk population.
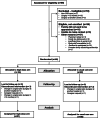
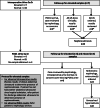
Methods: We conducted a two-arm, double-blind dose evaluation randomized controlled trial in children requiring cardiopulmonary bypass for congenital heart disease. Pre-operatively, participants were randomized to receive cholecalciferol representing usual care (< 1 year = 400 IU/day, > 1 year = 600 IU/day) or a higher dose approximating the Institute of Medicine tolerable upper intake level (< 1 year = 1600 IU/day, > 1 year = 2400 IU/day). The feasibility outcomes were post-operative vitamin D status (primary), vitamin D-related adverse events, accrual rate, study withdrawal rate, blinding, and protocol non-adherence.
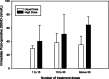
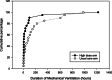
Results: Forty-six children were randomized, and five withdrew prior to surgery, leaving 41 children (21 high dose, 20 usual care) in the final analysis. The high dose group had higher 25-hydroxyvitamin D concentrations both intraoperatively (mean difference + 25.9 nmol/L; 95% CI 8.3-43.5) and post-operatively (mean difference + 17.2 nmol/L; 95% CI 5.5-29.0). Fewer participants receiving high-dose supplementation had post-operative serum 25-hydroxyvitamin D concentrations under 50 nmol/L, compared with usual care (RR 0.31, 95% CI 0.11-0.87). Post-operative vitamin D status was associated with the treatment arm and the number of doses received. There were no cases of hypercalcemia, and no significant adverse events related to vitamin D. While only 75% of the target sample size was recruited (limited funding), the consent rate (83%), accrual rate (1.5 per site month), number of withdrawals (11%), and ability to maintain blinding support feasibility of a larger trial.
Conclusions: Pre-operative daily high-dose supplementation improved vitamin D status pre-operatively and at time of pediatric ICU admission. The protocol for a more definitive trial should limit enrollment of children with at least 30 days between randomization and surgery to allow adequate duration of supplementation or consider a loading dose.
Trial registration: ClinicalTrials.gov, NCT01838447. Registered on April 24, 2013.
Keywords: Cholecalciferol; Congenital heart disease; Critical care; Dose evaluation trial; High-dose; Pediatric intensive care unit; Vitamin D deficiency.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsDr. Khamessan is employed by Euro-Pharm International Canada Inc. Dr. Khamessan and Euro-Pharm International Canada Inc., at the request of the investigative team, developed and prepared the concentrated vitamin D solution used in the study.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

