Transient Transfection of the Respiratory Epithelium with Gamma Interferon for Host-Directed Therapy in Pulmonary Tuberculosis
- PMID: 33110704
- PMCID: PMC7581375
- DOI: 10.1016/j.omtn.2020.10.023
Transient Transfection of the Respiratory Epithelium with Gamma Interferon for Host-Directed Therapy in Pulmonary Tuberculosis
Abstract
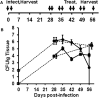
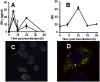
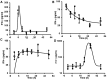
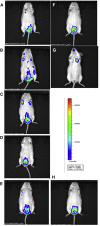
Nebulized gamma interferon (IFN-γ) protein has been studied for clinical safety and efficacy against pulmonary tuberculosis (TB). The protein is expensive, requires a cold chain, and is difficult to deploy in limited-resource, high-incidence settings. We generated a preclinical proof of concept (PoC) for a dry powder inhalation (DPI) containing DNA constructs to transiently transfect the lung and airway epithelium of mice with murine IFN-γ. Bacterial colony-forming units (CFU) in the lungs of mice infected with Mycobacterium tuberculosis (Mtb) reduced from about 106/g of tissue to ~104 after four doses given once a week. Nodular inflammatory lesions in the lungs reduced significantly in number. Immunohistochemistry of infected lung sections for LC3-1 and LAMP-1 indicated autophagy induction between 18 and 48 h after inhalation. ELISA on bronchoalveolar lavage (BAL) fluid showed differences in kinetics of IFN-γ concentrations in the epithelial lining fluid of healthy versus infected mice. Uninfected mice receiving DNA constructs expressing a fluorescent protein were live-imaged. The fluorescence signals from the intracellular protein peaked at about 36 h after inhalation and declined by 48 h. These results establish preclinical PoC of the efficacy of a DPI and dosing regimen as a host-directed and transient gene therapy of experimental pulmonary TB in mice, justifying preclinical development.
Keywords: dry powder inhalation; gamma interferon; gene delivery; gene therapy; host-directed therapy; preclinical proof of concept; pulmonary tuberculosis.
© 2020 The Authors.
Figures



Similar articles
-
Transient, inhaled gene therapy with gamma interferon mitigates pathology induced by host response in a mouse model of tuberculosis.Tuberculosis (Edinb). 2022 May;134:102198. doi: 10.1016/j.tube.2022.102198. Epub 2022 Mar 18. Tuberculosis (Edinb). 2022. PMID: 35344918
-
Preparation and Evaluation of Low-Dose Calcitriol Dry Powder Inhalation as Host-Directed Adjunct Therapy for Tuberculosis.Pharm Res. 2022 Oct;39(10):2621-2633. doi: 10.1007/s11095-022-03360-5. Epub 2022 Aug 12. Pharm Res. 2022. PMID: 35962268 Free PMC article.
-
Preparation and Preclinical Evaluation of Inhalable Particles Containing Rapamycin and Anti-Tuberculosis Agents for Induction of Autophagy.Pharm Res. 2016 Aug;33(8):1899-912. doi: 10.1007/s11095-016-1926-0. Epub 2016 Apr 19. Pharm Res. 2016. PMID: 27095353
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
Cited by
-
The Use of Viral Vectors for Gene Therapy and Vaccination in Tuberculosis.Pharmaceuticals (Basel). 2023 Oct 16;16(10):1475. doi: 10.3390/ph16101475. Pharmaceuticals (Basel). 2023. PMID: 37895946 Free PMC article. Review.
-
Overview of mucosal immunity and respiratory infections in children: a focus on Africa.Curr Opin Pediatr. 2025 Apr 1;37(2):137-144. doi: 10.1097/MOP.0000000000001438. Epub 2025 Feb 5. Curr Opin Pediatr. 2025. PMID: 39907513 Free PMC article. Review.
-
Atorvastatin Potentially Reduces Mycobacterial Severity through Its Action on Lipoarabinomannan and Drug Permeability in Granulomas.Microbiol Spectr. 2023 Jan 31;11(2):e0319722. doi: 10.1128/spectrum.03197-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36719189 Free PMC article.
-
Dominant negative biologics normalise the tumour necrosis factor (TNF-α) induced angiogenesis which exploits the Mycobacterium tuberculosis dissemination.BMC Immunol. 2023 Nov 30;24(1):49. doi: 10.1186/s12865-023-00576-x. BMC Immunol. 2023. PMID: 38036985 Free PMC article.
References
-
- Ernstoff M.S., Trautman T., Davis C.A., Reich S.D., Witman P., Balser J., Rudnick S., Kirkwood J.M. A randomized phase I/II study of continuous versus intermittent intravenous interferon gamma in patients with metastatic melanoma. J. Clin. Oncol. 1987;5:1804–1810. - PubMed
-
- Jaffe H.A., Buhl R., Mastrangeli A., Holroyd K.J., Saltini C., Czerski D., Jaffe H.S., Kramer S., Sherwin S., Crystal R.G. Organ specific cytokine therapy. Local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-gamma to the human lung. J. Clin. Invest. 1991;88:297–302. - PMC - PubMed
-
- Condos R., Hull F.P., Schluger N.W., Rom W.N., Smaldone G.C. Regional deposition of aerosolized interferon-γ in pulmonary tuberculosis. Chest. 2004;125:2146–2155. - PubMed
-
- Condos R., Rom W.N., Schluger N.W. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-γ via aerosol. Lancet. 1997;349:1513–1515. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

