In Vitro Anticancer Potential of Berberis lycium Royle Extracts against Human Hepatocarcinoma (HepG2) Cells
- PMID: 33110920
- PMCID: PMC7582056
- DOI: 10.1155/2020/8256809
In Vitro Anticancer Potential of Berberis lycium Royle Extracts against Human Hepatocarcinoma (HepG2) Cells
Abstract
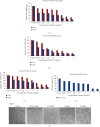
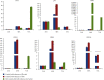
Human liver cancer has emerged as a serious health concern in the world, associated with poorly available therapies. The Berberis genus contains vital medicinal plants with miraculous healing properties and a wide range of bioactivities. In this study, different crude extracts of B. lycium Royle were prepared and screened against Human Hepatocarcinoma (HepG2) cell lines. The water/ethanolic extract of B. lycium Royle (BLE) exhibited significant antiproliferative activity against the HepG2 cancer cell line with an IC50 value of 47 μg/mL. The extract decreased the clonogenic potential of HepG2 cells in a dose-dependent manner. It induced apoptotic cell death in HepG2 cells that were confirmed by cytometric analysis and microscopic examination of cellular morphology through DAPI-stained cells. Biochemical evidence of apoptosis came from elevating the intracellular ROS level that was accompanied by the loss of mitochondrial membrane potential. The mechanism of apoptosis was further confirmed by gene expression analysis using RT-qPCR that revealed the decline in Bcl-2 independent of p53 mRNA and a rise in CDK1 while downregulating CDK5, CDK9, and CDK10 mRNA levels at 48 h of BLE treatment. The most active fraction was subjected to HPLC which indicated the presence of berberine (48 μg/mL) and benzoic acid (15.8 μg/mL) as major compounds in BLE and a trace amount of luteolin, rutin, and gallic acid. Our study highlighted the importance of the most active BLE extract as an excellent source of nutraceuticals against Human Hepatocarcinoma that can serve as an herbal natural cure against liver cancer.
Copyright © 2020 Kiren Mustafa et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



References
-
- Arayne M. S., Sultana N., Bahadur S. S. The Berberis story: Berberis vulgaris in therapeutics. Pakistan Journal of Pharmaceutical Sciences. 2007;20(1):83–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

