Microenvironment Stimulated Bioresponsive Small Molecule Carriers for Radiopharmaceuticals
- PMID: 33110957
- PMCID: PMC7581084
- DOI: 10.1021/acsomega.0c03601
Microenvironment Stimulated Bioresponsive Small Molecule Carriers for Radiopharmaceuticals
Abstract
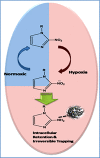
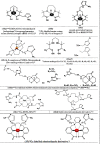
The widespread and successful use of radiopharmaceuticals in diagnosis, treatment, and therapeutic monitoring of cancer and other ailments has spawned significant literature. The transition from untargeted to targeted radiopharmaceuticals reflects the various stages of design and development. Targeted radiopharmaceuticals bind to specific biomarkers, get fixed, and highlight the disease site. A new subset of radioprobes, the bioresponsive radiopharmaceuticals, has been developed in recent years. These probes generally benefit from signal enhancement after undergoing molecular changes due to the fluctuations in the environment (pH, redox, or enzymatic activity) at the site of interest. This review presents a comprehensive overview of bioresponsive radioimaging probes covering the basis, application, and scope of development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
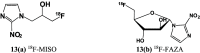

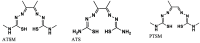

References
-
- van Duijnhoven S. M.; Robillard M. S.; Langereis S.; Grüll H. Bioresponsive probes for molecular imaging: concepts and in vivo applications. Contrast Media Mol. Imaging 2015, 10 (4), 282–308. 10.1002/cmmi.1636. - DOI - PubMed
- Chaturvedi S.; Kaul A.; Hazari P. P.; Mishra A. K. Mapping neuroreceptors with metal-labeled radiopharmaceuticals. MedChemComm 2017, 8 (5), 855–870. 10.1039/C6MD00610H. - DOI - PMC - PubMed
- Chaturvedi S.; Mishra A. K. Small molecule radiopharmaceuticals–a review of current approaches. Front. Med. 2016, 3, 5. 10.3389/fmed.2016.00005. - DOI - PMC - PubMed
-
- Cho M. H.; Shin S. H.; Park S. H.; Kadayakkara D. K.; Kim D.; Choi Y. Targeted, Stimuli-Responsive, and Theranostic 19F Magnetic Resonance Imaging Probes. Bioconjugate Chem. 2019, 30 (10), 2502–2518. 10.1021/acs.bioconjchem.9b00582. - DOI - PubMed
- Pinto S. M.; Tomé V.; Calvete M. J.; Castro M. M. C.; Tóth É.; Geraldes C. F. Metal-based redox-responsive MRI contrast agents. Coord. Chem. Rev. 2019, 390, 1–31. 10.1016/j.ccr.2019.03.014. - DOI
-
- Hunt J. F.; Rath P.; Rothschild K. J.; Engelman D. M. Spontaneous, pH-dependent membrane insertion of a transbilayer α-helix. Biochemistry 1997, 36 (49), 15177–15192. 10.1021/bi970147b. - DOI - PubMed
- Va̅vere A. L.; Biddlecombe G. B.; Spees W. M.; Garbow J. R.; Wijesinghe D.; Andreev O. A.; Engelman D. M.; Reshetnyak Y. K.; Lewis J. S. A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res. 2009, 69 (10), 4510–4516. 10.1158/0008-5472.CAN-08-3781. - DOI - PMC - PubMed
- Viola-Villegas N. T.; Carlin S. D.; Ackerstaff E.; Sevak K. K.; Divilov V.; Serganova I.; Kruchevsky N.; Anderson M.; Blasberg R. G.; Andreev O. A.; Engelman D. M.; Koutcher J. A.; Reshetnyak Y. K.; Lewis J. S. Understanding the pharmacological properties of a metabolic PET tracer in prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (20), 7254–7259. 10.1073/pnas.1405240111. - DOI - PMC - PubMed
- Demoin D. W.; Wyatt L. C.; Edwards K. J.; Abdel-Atti D.; Sarparanta M.; Pourat J.; Longo V. A.; Carlin S. D.; Engelman D. M.; Andreev O. A.; Reshetnyak Y. K.; Viola-Villegas N.; Lewis J. S. PET imaging of extracellular pH in tumors with 64Cu-and 18F-labeled pHLIP peptides: a structure–activity optimization study. Bioconjugate Chem. 2016, 27 (9), 2014–2023. 10.1021/acs.bioconjchem.6b00306. - DOI - PMC - PubMed
- Macholl S.; Morrison M. S.; Iveson P.; Arbo B. E.; Andreev O. A.; Reshetnyak Y. K.; Engelman D. M.; Johannesen E. In vivo pH imaging with 99m Tc-pHLIP. Mol. Imaging Biol. 2012, 14 (6), 725–734. 10.1007/s11307-012-0549-z. - DOI - PMC - PubMed
- Daumar P.; Wanger-Baumann C. A.; Pillarsetty N.; Fabrizio L.; Carlin S. D.; Andreev O. A.; Reshetnyak Y. K.; Lewis J. S. Efficient 18F-labeling of large 37-amino-acid pHLIP peptide analogues and their biological evaluation. Bioconjugate Chem. 2012, 23 (8), 1557–1566. 10.1021/bc3000222. - DOI - PMC - PubMed
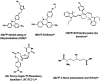
- Flavell R. R.; Truillet C.; Regan M. K.; Ganguly T.; Blecha J. E.; Kurhanewicz J.; VanBrocklin H. F.; Keshari K. R.; Chang C. J.; Evans M. J.; Wilson D. M. Caged [18F] FDG Glycosylamines for Imaging Acidic Tumor Microenvironments Using Positron Emission Tomography. Bioconjugate Chem. 2016, 27 (1), 170–178. 10.1021/acs.bioconjchem.5b00584. - DOI - PMC - PubMed
-
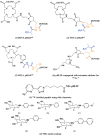
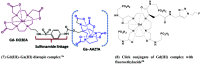
- Vologdin N.; Rolla G. A.; Botta M.; Tei L. Orthogonal synthesis of a heterodimeric ligand for the development of the Gd III–Ga III ditopic complex as a potential pH-sensitive MRI/PET probe. Org. Biomol. Chem. 2013, 11 (10), 1683–1690. 10.1039/c2ob27200h. - DOI - PubMed
- Frullano L.; Catana C.; Benner T.; Sherry A. D.; Caravan P. Bimodal MR–PET agent for quantitative pH imaging. Angew. Chem., Int. Ed. 2010, 49 (13), 2382–2384. 10.1002/anie.201000075. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

