Hydrocracking of Fischer-Tropsch Paraffin Mixtures over Strong Acid Bifunctional Catalysts to Engine Fuels
- PMID: 33110969
- PMCID: PMC7581076
- DOI: 10.1021/acsomega.0c02711
Hydrocracking of Fischer-Tropsch Paraffin Mixtures over Strong Acid Bifunctional Catalysts to Engine Fuels
Abstract
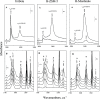
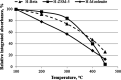
Biomass-based Fischer-Tropsch paraffin mixtures, having C16-C46 and C11-C45 carbon number range, were hydrocracked over platinum-supported beta, ZSM-5, and mordenite catalysts. The aim of the study was to investigate the effects of the feedstock composition, the process parameters, and the catalyst properties (acidity and zeolite structure) on the C21+ conversions, the product yields, and the isoparaffin contents. It was stated that lower C21+ conversions and higher JET and diesel fuel yields can be obtained for feedstock comprising C11-C45 hydrocarbons. Under identical reaction conditions, the activity order of the catalysts was Pt/H-beta > Pt/H-ZSM-5 > Pt/H-mordenite. This order corresponds to the relative number of accessible acid sites. Among the tested catalysts, platinum-supported beta zeolite showed the highest hydroisomerization activity; meanwhile, in the pores of Pt/ZSM-5 and Pt/H-mordenite, diffusion constraints were observed. As the product of hydrocracking valuable gasoline, JET and diesel fuels having high hydrogen content and excellent burning properties were produced.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- BP, Energy Outlook 2018 edition. https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/ener... (accessed May, 25 2020).
-
- ExxonMobil . Outlook for Energy: A view to 2040. https://corporate.exxonmobil.com/en/energy/energy-outlook/a-view-to-2040 (accessed May, 25 2020).
-
- Joshi G.; Pandey J. K.; Rana S.; Rawat D. S. Challenges and opportunities for the application of biofuel. Renewable Sustainable Energy Rev. 2017, 79, 850–866. 10.1016/j.rser.2017.05.185. - DOI
-
- Bregthorson J. M.; Thomson M. J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renewable Sustainable Energy Rev. 2015, 42, 1393–1417. 10.1016/j.rser.2014.10.034. - DOI
-
- European Biogas Association (EBA) . http://european-biogas.eu/wp-content/uploads/2018/07/REDII-Summary_EBA.pdf (accessed 25 May 2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

