High-Throughput Mass Cytometry Staining for Immunophenotyping Clinical Samples
- PMID: 33111099
- PMCID: PMC7580234
- DOI: 10.1016/j.xpro.2020.100055
High-Throughput Mass Cytometry Staining for Immunophenotyping Clinical Samples
Abstract
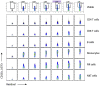
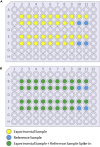
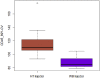
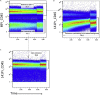
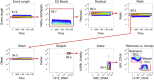
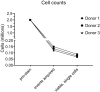
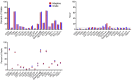
As mass cytometry (MC) is implemented in clinical settings, the need for robust, validated protocols that reduce batch effects between samples becomes increasingly important. Here, we present a streamlined MC workflow for high-throughput staining that generates reproducible data for up to 80 samples in a single experiment by combining reference sample spike-in and palladium-based mass-tag cell barcoding. Although labor intensive, this workflow decreases experimental variables and thus reduces technical error and mitigates batch effects.
© 2020 The Authors.
Conflict of interest statement
K.K. is currently an employee of bluebird bio. F.S.H. serves as a consultant to Genentech, Bristol-Myers Squibb, Merck, Novartis, Amgen, Sanofi, Bayer, Pfizer, EMD Serono, Verastem, Aduro, Celldex, and Incyte.
Figures


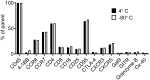



References
-
- Bagwell C.B., Inokuma M., Hunsberger B., Herbert D., Bray C., Hill B., Stelzer G., LI S., Kollipara A., Ornatsky O. Automated data cleanup for mass cytometry. Cytometry A. 2020;97:184–198. - PubMed
-
- Holland M., Cunningham R., Seymour L., Kleinsteuber K., Cunningham A., Patel T., Manos M., Brennick R., Zhou J., Hodi F.S. Separation, banking, and quality control of peripheral blood mononuclear cells from whole blood of melanoma patients. Cell Tissue Bank. 2018;19:783–790. - PubMed
-
- Lee B.H., Kelly G., Bradford S., Davila M., Guo X.V., Amir E.D., Thrash E.M., Solga M.D., Lannigan J., Sellers B. A modified injector and sample acquisition protocol can improve data quality and reduce inter-instrument variability of the helios mass cytometer. Cytometry A. 2019;95:1019–1030. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

