Protocol for Primary Mouse Hepatocyte Isolation
- PMID: 33111119
- PMCID: PMC7580103
- DOI: 10.1016/j.xpro.2020.100086
Protocol for Primary Mouse Hepatocyte Isolation
Abstract
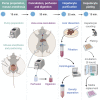
Primary hepatocytes are a vital tool in various biomedical research disciplines, serving as an ex vivo model for liver physiology. Obtaining high yields of viable primary mouse hepatocytes is technically challenging, limiting their use. Here, we present an improved protocol based on the classic two-step collagenase perfusion technique. The liver is washed by perfusion, hepatocytes are dissociated by collagenase, separated from other cells, and cultured. This protocol was optimized to significantly reduce procedure duration and improve hepatocyte yield and viability.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Azimifar S.B., Nagaraj N., Cox J., Mann M. Cell-type-resolved quantitative proteomics of murine liver. Cell Metab. 2014;20:1076–1087. - PubMed
-
- Casciano D.A. Development and utilization of primary hepatocyte culture systems to evaluate metabolism, DNA binding, and DNA repair of xenobiotics. Drug Metab. Rev. 2000;32:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

