Bacillus Calmette-Guerin (BCG) Vaccine-associated Complications in Immunodeficient Patients Following Stem Cell Transplantation
- PMID: 33111199
- PMCID: PMC7591244
- DOI: 10.1007/s10875-020-00892-6
Bacillus Calmette-Guerin (BCG) Vaccine-associated Complications in Immunodeficient Patients Following Stem Cell Transplantation
Abstract
Purpose: Bacillus Calmette-Guerin (BCG) is a live attenuated vaccine with the potential of causing severe iatrogenic complications in patients with primary immunodeficiency diseases (PID) before and after hematopoietic stem cell transplantation (HSCT). We aim to investigate risk factors of post-HSCT BCG-related complications in PID patients.
Methods: A retrospective analysis of pediatric PID patients who had received the BCG vaccine and underwent HSCT at Hadassah-Hebrew University Medical Center, between 2007 and 2019.
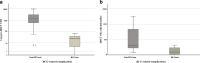
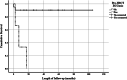
Results: We found 15/36 (41.67%) patients who developed post-HSCT BCG-related complications. The most significant risk factor for developing BCG-related complications was T cell deficiency (47.6% of the non-complicated vs 83.3% of the BCGitis and 100% of the BCGosis groups had T cell lymphopenia, p = 0.013). None of the chronic granulomatous patients developed BCG-related manifestation post-transplant. Among T cell-deficient patients, lower NK (127 vs 698 cells/μl, p = 0.04) cell counts and NK-SCID were risk factors for ongoing post-HSCT BCGosis, as was pretransplant disseminated BCGosis (33.3% of patients with BCGosis vs none of the non-BCGosis patients, p = 0.04). Immune reconstitution inflammatory syndrome (IRIS) was observed in 3/5 patients with Omenn syndrome. Prophylactic antimycobacterial treatment was not proven effective.
Conclusion: BCG vaccination can cause significant morbidity and mortality in the post-transplant T cell-deficient patient, especially in the presence of pre-transplant disease. Taking a detailed medical history prior to administering, the BCG vaccine is crucial for prevention of this complication.
Keywords: BCG vaccination; hematopoietic stem cell transplantation; immune reconstitution inflammatory syndrome; primary immune deficiency; severe combined immunodeficiency.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Evaluation of patients with primary immunodeficiency associated with Bacille Calmette-Guerin (BCG)-vaccine-derived complications.Allergol Immunopathol (Madr). 2020 Nov-Dec;48(6):729-737. doi: 10.1016/j.aller.2020.04.004. Epub 2020 Oct 25. Allergol Immunopathol (Madr). 2020. PMID: 33115608
-
DISSEMINATED BACILLUS-CALMETTE-GUÉRIN INFECTIONS AND PRIMARY IMMUNODEFICIENCY DISORDERS IN SINGAPORE: A SINGLE CENTER 15-YEAR RETROSPECTIVE REVIEW.Int J Infect Dis. 2020 Aug;97:117-125. doi: 10.1016/j.ijid.2020.05.117. Epub 2020 Jun 2. Int J Infect Dis. 2020. PMID: 32497805 Review.
-
Effect of Early Bacillus Calmette-Guerin Vaccination of Pediatric Severe Combined Immunodeficiency Patients on the Outcome of Hematopoietic Stem Cell Transplantation Using a Reduced-Intensity Conditioning Regimen.Transplant Cell Ther. 2023 Mar;29(3):188.e1-188.e8. doi: 10.1016/j.jtct.2022.12.007. Epub 2022 Dec 17. Transplant Cell Ther. 2023. PMID: 36539079
-
Differential diagnosis of primary immunodeficiency in patients with BCGitis and BCGosis: A single-centre study.Scand J Immunol. 2021 Oct;94(4):e13084. doi: 10.1111/sji.13084. Epub 2021 Jul 28. Scand J Immunol. 2021. PMID: 34780073
-
Primary Immunodeficiency Diseases and Bacillus Calmette-Guérin (BCG)-Vaccine-Derived Complications: A Systematic Review.J Allergy Clin Immunol Pract. 2020 Apr;8(4):1371-1386. doi: 10.1016/j.jaip.2020.01.038. Epub 2020 Jan 30. J Allergy Clin Immunol Pract. 2020. PMID: 32006723
Cited by
-
Granulomatous inflammation in inborn errors of immunity.Front Pediatr. 2023 Feb 20;11:1110115. doi: 10.3389/fped.2023.1110115. eCollection 2023. Front Pediatr. 2023. PMID: 36891233 Free PMC article. Review.
-
BCGitis as the primary manifestation of chronic granulomatous disease.IDCases. 2020 Dec 29;23:e01038. doi: 10.1016/j.idcr.2020.e01038. eCollection 2021. IDCases. 2020. PMID: 33425681 Free PMC article.
-
Prevalence and Characteristics of Non-tuberculous Mycobacteria (NTM) Infection in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation: a Systematic Review and Meta-analysis.J Clin Immunol. 2023 Dec 22;44(1):23. doi: 10.1007/s10875-023-01615-3. J Clin Immunol. 2023. PMID: 38129624 Free PMC article.
-
Bacillus Calmette-Guérin (BCG) prostato-epididymitis in a patient treated for a non-invasive urothelial cancer: A case report.IDCases. 2024 Apr 19;36:e01967. doi: 10.1016/j.idcr.2024.e01967. eCollection 2024. IDCases. 2024. PMID: 38699528 Free PMC article.
-
Unmasking Bacillus Calmette-Guérin Immune Reconstitution Inflammatory Syndrome in a Perinatal HIV Transmission-A Case Report.Trop Med Infect Dis. 2025 May 23;10(6):148. doi: 10.3390/tropicalmed10060148. Trop Med Infect Dis. 2025. PMID: 40559715 Free PMC article.
References
-
- WHO. Global tuberculosis report 2019. WHO; 2020
-
- Liu J, Tran V, Leung AS, Alexander DC, Zhu B. BCG vaccines: Their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccin. 2009;5:70–78. - PubMed
-
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, Gie RP, Cotton MF, van Helden PD, Warren RM, Beyers N. Bacille Calmette-Guerin vaccine–induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42(4):548–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

