Cisatracurium stimulates testosterone synthesis in rat and mouse Leydig cells via nicotinic acetylcholine receptor
- PMID: 33111502
- PMCID: PMC7754058
- DOI: 10.1111/jcmm.16029
Cisatracurium stimulates testosterone synthesis in rat and mouse Leydig cells via nicotinic acetylcholine receptor
Abstract
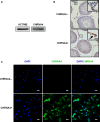
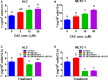
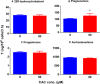
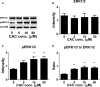
As a cis-acting non-depolarizing neuromuscular blocker through a nicotinic acetylcholine receptor (nAChR), cisatracurium (CAC) is widely used in anaesthesia and intensive care units. nAChR may be present on Leydig cells to mediate the action of CAC. Here, by Western blotting, immunohistochemistry and immunofluorescence, we identified that CHRNA4 (a subunit of nAChR) exists only on rat adult Leydig cells. We studied the effect of CAC on the synthesis of testosterone in rat adult Leydig cells and mouse MLTC-1 tumour cells. Rat Leydig cells and MLTC-1 cells were treated with CAC (5, 10 and 50 μmol/L) or nAChR agonists (50 μmol/L nicotine or 50 μmol/L lobeline) for 12 hours, respectively. We found that CAC significantly increased testosterone output in rat Leydig cells and mouse MLTC-1 cells at 5 μmol/L and higher concentrations. However, nicotine and lobeline inhibited testosterone synthesis. CAC increased intracellular cAMP levels, and nicotine and lobeline reversed this change in rat Leydig cells. CAC may increase testosterone synthesis in rat Leydig cells and mouse MLTC-1 cells by up-regulating the expression of Lhcgr and Star. Up-regulation of Scarb1 and Hsd3b1 expression by CAC was also observed in rat Leydig cells. In addition to cAMP signal transduction, CAC can induce ERK1/2 phosphorylation in rat Leydig cells. In conclusion, CAC binds to nAChR on Leydig cells, and activates cAMP and ERK1/2 phosphorylation, thereby up-regulating the expression of key genes and proteins in the steroidogenic cascade, resulting in increased testosterone synthesis in Leydig cells.
Keywords: CHRNA4; MLTC-1 cells; adult Leydig cells; cisatracurium; nAChR; testosterone.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest to declare.
Figures



Similar articles
-
Expression patterns of C1ql4 and its cell-adhesion GPCR Bai3 in the murine testis and functional roles in steroidogenesis.FASEB J. 2019 Apr;33(4):4893-4906. doi: 10.1096/fj.201801620RR. Epub 2019 Jan 4. FASEB J. 2019. PMID: 30608882
-
Nicotinic cholinergic agonists inhibit androgen biosynthesis by cultured rat testicular cells.Endocrinology. 1985 Nov;117(5):1874-80. doi: 10.1210/endo-117-5-1874. Endocrinology. 1985. PMID: 2864237
-
Leukemia inhibitory factor antagonizes gonadotropin induced-testosterone synthesis in cultured porcine leydig cells: sites of action.Endocrinology. 2001 Jun;142(6):2509-20. doi: 10.1210/endo.142.6.8177. Endocrinology. 2001. PMID: 11356700
-
Multistep regulation of Leydig cell function.J Steroid Biochem. 1987;27(1-3):343-50. doi: 10.1016/0022-4731(87)90326-8. J Steroid Biochem. 1987. PMID: 2826891 Review.
-
Hormonal regulation of steroidogenic enzyme gene expression in Leydig cells.J Steroid Biochem Mol Biol. 1992 Dec;43(8):895-906. doi: 10.1016/0960-0760(92)90317-C. J Steroid Biochem Mol Biol. 1992. PMID: 22217834 Review.
Cited by
-
The Smoky Impact of Nicotinic Acetylcholine Receptors on Testicular Function.J Clin Med. 2024 Aug 28;13(17):5097. doi: 10.3390/jcm13175097. J Clin Med. 2024. PMID: 39274310 Free PMC article. Review.
-
Natural Astaxanthin Improves Testosterone Synthesis and Sperm Mitochondrial Function in Aging Roosters.Antioxidants (Basel). 2022 Aug 29;11(9):1684. doi: 10.3390/antiox11091684. Antioxidants (Basel). 2022. PMID: 36139758 Free PMC article.
References
-
- Changeux JP, Corringer PJ, Maskos U. The nicotinic acetylcholine receptor: from molecular biology to cognition. Neuropharmacology. 2015;96:135‐136. - PubMed
-
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurogibol. 2004;74:363‐396. - PubMed
-
- Ge RS, Dong Q, Sottas CM, Chen H, Zirkin BR, Hardy MP. Gene expression in rat leydig cells during development from the progenitor to adult stage: a cluster analysis. Biol Reprod. 2005;72:1405‐1415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

