Functional exploration of heterotrimeric kinesin-II in IFT and ciliary length control in Chlamydomonas
- PMID: 33112235
- PMCID: PMC7652414
- DOI: 10.7554/eLife.58868
Functional exploration of heterotrimeric kinesin-II in IFT and ciliary length control in Chlamydomonas
Abstract
Heterodimeric motor organization of kinesin-II is essential for its function in anterograde IFT in ciliogenesis. However, the underlying mechanism is not well understood. In addition, the anterograde IFT velocity varies significantly in different organisms, but how this velocity affects ciliary length is not clear. We show that in Chlamydomonas motors are only stable as heterodimers in vivo, which is likely the key factor for the requirement of a heterodimer for IFT. Second, chimeric CrKinesin-II with human kinesin-II motor domains functioned in vitro and in vivo, leading to a ~ 2.8 fold reduced anterograde IFT velocity and a similar fold reduction in IFT injection rate that supposedly correlates with ciliary assembly activity. However, the ciliary length was only mildly reduced (~15%). Modeling analysis suggests a nonlinear scaling relationship between IFT velocity and ciliary length that can be accounted for by limitation of the motors and/or its ciliary cargoes, e.g. tubulin.
Keywords: C. elegans; Kinesin-II; cell biology; chlamydomonas reinhardtii; cilia; ciliary length; flagella; intraflagellar transport.
© 2020, Li et al.
Conflict of interest statement
SL, KW, WC, HT, XL No competing interests declared, JP Reviewing editor, eLife
Figures
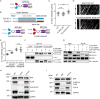
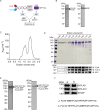







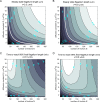
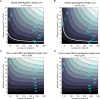

Similar articles
-
IFT54 directly interacts with kinesin-II and IFT dynein to regulate anterograde intraflagellar transport.EMBO J. 2021 Mar 1;40(5):e105781. doi: 10.15252/embj.2020105781. Epub 2020 Dec 28. EMBO J. 2021. PMID: 33368450 Free PMC article.
-
Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons.J Cell Biol. 1998 May 18;141(4):993-1008. doi: 10.1083/jcb.141.4.993. J Cell Biol. 1998. PMID: 9585417 Free PMC article.
-
Kinesin-2 from C. reinhardtii Is an Atypically Fast and Auto-inhibited Motor that Is Activated by Heterotrimerization for Intraflagellar Transport.Curr Biol. 2020 Mar 23;30(6):1160-1166.e5. doi: 10.1016/j.cub.2020.01.046. Epub 2020 Mar 5. Curr Biol. 2020. PMID: 32142698 Free PMC article.
-
Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery.FEBS J. 2017 Sep;284(18):2905-2931. doi: 10.1111/febs.14068. Epub 2017 Apr 18. FEBS J. 2017. PMID: 28342295 Free PMC article. Review.
-
The intraflagellar transport machinery of Chlamydomonas reinhardtii.Traffic. 2003 Jul;4(7):435-42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. Traffic. 2003. PMID: 12795688 Review.
Cited by
-
Regulation of kinesin-2 motility by its β-hairpin motif.Nat Struct Mol Biol. 2025 Jul 29. doi: 10.1038/s41594-025-01630-5. Online ahead of print. Nat Struct Mol Biol. 2025. PMID: 40730907
-
Assembly and stability of IFT-B complex and its function in BBSome trafficking.iScience. 2022 Nov 4;25(12):105493. doi: 10.1016/j.isci.2022.105493. eCollection 2022 Dec 22. iScience. 2022. PMID: 36411782 Free PMC article.
-
The flagellar length control system: exploring the physical biology of organelle size.Phys Biol. 2023 Jan 24;20(2):10.1088/1478-3975/acb18d. doi: 10.1088/1478-3975/acb18d. Phys Biol. 2023. PMID: 36623317 Free PMC article. Review.
-
Ciliary transition zone proteins coordinate ciliary protein composition and ectosome shedding.Nat Commun. 2022 Jul 9;13(1):3997. doi: 10.1038/s41467-022-31751-0. Nat Commun. 2022. PMID: 35810181 Free PMC article.
-
Mechanisms of Regulation in Intraflagellar Transport.Cells. 2022 Sep 2;11(17):2737. doi: 10.3390/cells11172737. Cells. 2022. PMID: 36078145 Free PMC article. Review.
References
-
- Bertiaux E, Mallet A, Fort C, Blisnick T, Bonnefoy S, Jung J, Lemos M, Marco S, Vaughan S, Trépout S, Tinevez JY, Bastin P. Bidirectional intraflagellar transport is restricted to two sets of microtubule doublets in the trypanosome flagellum. Journal of Cell Biology. 2018a;217:4284–4297. doi: 10.1083/jcb.201805030. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2017YFA0503500/Ministry of Science and Technology of the People's Republic of China/International
- 2018YFA0902500/Ministry of Science and Technology of the People's Republic of China/International
- 31991191/National Natural Science Foundation of China/International
- 31671387/National Natural Science Foundation of China/International
- 31972888/National Natural Science Foundation of China/International
- 31922018/National Natural Science Foundation of China/International
- SBF003\1160/Springboard Award from the Academy of Medical Sciences and Global Challenges Research Fund Research Fund/International
- SBF003\1160/Springboard Award from the Academy of Medical Sciences and Global Challenges Research Fund/International
LinkOut - more resources
Full Text Sources

