Low-Dose Total Skin Electron Beam Therapy as Part of a Multimodality Regimen for Treatment of Sézary Syndrome: Clinical, Immunologic, and Molecular Analysis
- PMID: 33112366
- PMCID: PMC7593882
- DOI: 10.1001/jamadermatol.2020.3958
Low-Dose Total Skin Electron Beam Therapy as Part of a Multimodality Regimen for Treatment of Sézary Syndrome: Clinical, Immunologic, and Molecular Analysis
Abstract
Importance: Sézary syndrome (SS) is an advanced form of cutaneous T-cell lymphoma with few long-term remissions observed.
Objective: To profile 3 patients with SS who have experienced long-term remission following the addition of low-dose total skin electron beam therapy (TSEBT) to systemic regimens of extracorporeal photopheresis, bexarotene, and interferon-γ.
Design, setting, and participants: This is a retrospective case series with additional investigations of patient-donated samples to assess therapeutic response. The study was conducted at the University of Pennsylvania Cutaneous Lymphoma Clinic and follows 3 patients with stage IVA1 CD4+ SS who presented to the clinic between November 1, 2009, and November 1, 2017, and who had a history of SS that was refractory to multimodality systemic therapy prior to receiving low-dose TSEBT.
Interventions: Patients were treated in a multimodality fashion with combined extracorporeal photopheresis, bexarotene, interferon-γ, and low-dose TSEBT.
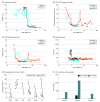
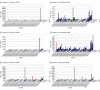
Main outcomes and measures: To characterize treatment responses in these patients, the extent of skin disease was measured with the modified severity weighted assessment tool. Blood disease was measured with flow cytometric assessments of Sézary cell count, CD4:CD8 ratio, and high throughput sequencing of the T-cell receptors. To assess for restoration of immune function, we measured markers of immune exhaustion, including PD-1 (programmed cell death 1), TIGIT (T-cell immunoreceptor with immunoglobulin and ITIM domains), CTLA4 (cytotoxic T-lymphocyte-associated protein 4), TOX (thymocyte selection-associated high mobility group box protein), and Foxp3 (forkhead box P3) on circulating CD4 and CD8 T cells, along with production capacity of interferon-γ by lymphocytes following activation stimuli.
Results: Following administration of low-dose TSEBT and maintenance of the other therapies, remissions ranged from 24 to 30 months, with complete responses in 2 patients ongoing. Markers of immune exhaustion including PD-1, TIGIT, CTLA4, TOX, and Foxp3 were significantly reduced from baseline following TSEBT, along with enhanced production capacity of interferon-γ by lymphocytes following activation stimuli. High throughput sequencing demonstrated near-complete eradication of the circulating clone among 2 of 3 patients with stable levels in 1.
Conclusions and relevance: We describe 3 patients who achieved long-term clinical and molecular remissions following low-dose TSEBT as part of a multimodality regimen for treatment of SS. As long-term remissions in SS are uncommon, this approach demonstrates promise, and clinical trials should be considered.
Conflict of interest statement
Figures
References
-
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730-4739. doi: 10.1200/JCO.2009.27.7665 - DOI - PubMed
-
- Stadler R, Otte HG, Luger T, et al. Prospective randomized multicenter clinical trial on the use of interferon -2a plus acitretin versus interferon -2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood. 1998;92(10):3578-3581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

