Development and Validation of a Comprehensive Model to Estimate Early Allograft Failure Among Patients Requiring Early Liver Retransplant
- PMID: 33112390
- PMCID: PMC7593884
- DOI: 10.1001/jamasurg.2020.4095
Development and Validation of a Comprehensive Model to Estimate Early Allograft Failure Among Patients Requiring Early Liver Retransplant
Erratum in
-
Missing Degrees in Byline and Errors in Equation.JAMA Surg. 2021 Jan 1;156(1):105. doi: 10.1001/jamasurg.2020.5513. JAMA Surg. 2021. PMID: 33175101 Free PMC article. No abstract available.
Abstract
Importance: Expansion of donor acceptance criteria for liver transplant increased the risk for early allograft failure (EAF), and although EAF prediction is pivotal to optimize transplant outcomes, there is no consensus on specific EAF indicators or timing to evaluate EAF. Recently, the Liver Graft Assessment Following Transplantation (L-GrAFT) algorithm, based on aspartate transaminase, bilirubin, platelet, and international normalized ratio kinetics, was developed from a single-center database gathered from 2002 to 2015.
Objective: To develop and validate a simplified comprehensive model estimating at day 10 after liver transplant the EAF risk at day 90 (the Early Allograft Failure Simplified Estimation [EASE] score) and, secondarily, to identify early those patients with unsustainable EAF risk who are suitable for retransplant.
Design, setting, and participants: This multicenter cohort study was designed to develop a score capturing a continuum from normal graft function to nonfunction after transplant. Both parenchymal and vascular factors, which provide an indication to list for retransplant, were included among the EAF determinants. The L-GrAFT kinetic approach was adopted and modified with fewer data entries and novel variables. The population included 1609 patients in Italy for the derivation set and 538 patients in the UK for the validation set; all were patients who underwent transplant in 2016 and 2017.
Main outcomes and measures: Early allograft failure was defined as graft failure (codified by retransplant or death) for any reason within 90 days after transplant.
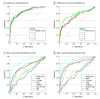
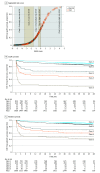
Results: At day 90 after transplant, the incidence of EAF was 110 of 1609 patients (6.8%) in the derivation set and 41 of 538 patients (7.6%) in the external validation set. Median (interquartile range) ages were 57 (51-62) years in the derivation data set and 56 (49-62) years in the validation data set. The EASE score was developed through 17 entries derived from 8 variables, including the Model for End-stage Liver Disease score, blood transfusion, early thrombosis of hepatic vessels, and kinetic parameters of transaminases, platelet count, and bilirubin. Donor parameters (age, donation after cardiac death, and machine perfusion) were not associated with EAF risk. Results were adjusted for transplant center volume. In receiver operating characteristic curve analyses, the EASE score outperformed L-GrAFT, Model for Early Allograft Function, Early Allograft Dysfunction, Eurotransplant Donor Risk Index, donor age × Model for End-stage Liver Disease, and Donor Risk Index scores, estimating day 90 EAF in 87% (95% CI, 83%-91%) of cases in both the derivation data set and the internal validation data set. Patients could be stratified in 5 classes, with those in the highest class exhibiting unsustainable EAF risk.
Conclusions and relevance: This study found that the developed EASE score reliably estimated EAF risk. Knowledge of contributing factors may help clinicians to mitigate risk factors and guide them through the challenging clinical decision to allocate patients to early liver retransplant. The EASE score may be used in translational research across transplant centers.
Conflict of interest statement
Figures

Comment in
-
Predicting Early Hepatic Graft Failure-The Quest Continues.JAMA Surg. 2020 Dec 1;155(12):e204593. doi: 10.1001/jamasurg.2020.4593. Epub 2020 Dec 16. JAMA Surg. 2020. PMID: 33112384 No abstract available.
References
-
- Deschênes M, Belle SH, Krom RA, Zetterman RK, Lake JR; National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database . Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. Transplantation. 1998;66(3):302-310. doi:10.1097/00007890-199808150-00005 - DOI - PubMed

