An in vitro assessment of the response of THP-1 macrophages to varying stiffness of a glycol-chitosan hydrogel for vocal fold tissue engineering applications
- PMID: 33112473
- PMCID: PMC8079542
- DOI: 10.1002/jbm.a.37125
An in vitro assessment of the response of THP-1 macrophages to varying stiffness of a glycol-chitosan hydrogel for vocal fold tissue engineering applications
Abstract
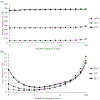
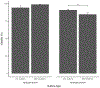
The physical properties of a biomaterial play an essential role in regulating immune and reparative activities within the host tissue. This study aimed to evaluate the immunological impact of material stiffness of a glycol-chitosan hydrogel designed for vocal fold tissue engineering. Hydrogel stiffness was varied via the concentration of glyoxal cross-linker applied. Hydrogel mechanical properties were characterized through atomic force microscopy and shear plate rheometry. Using a transwell setup, macrophages were co-cultured with human vocal fold fibroblasts that were embedded within the hydrogel. Macrophage viability and cytokine secretion were evaluated at 3, 24, and 72 hr of culture. Flow cytometry was applied to evaluate macrophage cell surface markers after 72 hr of cell culture. Results indicated that increasing hydrogel stiffness was associated with increased anti-inflammatory activity compared to relevant controls. In addition, increased anti-inflammatory activity was observed in hydrogel co-cultures. This study highlighted the importance of hydrogel stiffness from an immunological viewpoint when designing novel vocal fold hydrogels.
Keywords: fibroblast; hydrogel; immunomodulation; macrophage; stiffness.
© 2020 Wiley Periodicals LLC.
Figures


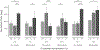
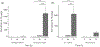
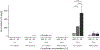

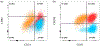
Similar articles
-
In Vitro Investigation of Vocal Fold Cellular Response to Variations in Hydrogel Porosity and Elasticity.ACS Biomater Sci Eng. 2024 Jun 10;10(6):3909-3922. doi: 10.1021/acsbiomaterials.4c00197. Epub 2024 May 24. ACS Biomater Sci Eng. 2024. PMID: 38783819
-
Cell and tissue responses at the interface with a chitosan hydrogel intended for vascular applications: in vitro and in vivo exploration.Biomed Mater. 2019 Jan 30;14(2):025009. doi: 10.1088/1748-605X/aafbf0. Biomed Mater. 2019. PMID: 30609413
-
3D printable and injectable lactoferrin-loaded carboxymethyl cellulose-glycol chitosan hydrogels for tissue engineering applications.Mater Sci Eng C Mater Biol Appl. 2020 Aug;113:111008. doi: 10.1016/j.msec.2020.111008. Epub 2020 Apr 24. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32487412
-
A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine.J Biomed Mater Res A. 2017 Jun;105(6):1799-1812. doi: 10.1002/jbm.a.36034. Epub 2017 Apr 3. J Biomed Mater Res A. 2017. PMID: 28187512 Review.
-
A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair.Front Bioeng Biotechnol. 2022 Jan 25;10:817391. doi: 10.3389/fbioe.2022.817391. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35145958 Free PMC article. Review.
Cited by
-
On the path to predicting immune responses in the lung: Modeling the pulmonary innate immune system at the air-liquid interface (ALI).Eur J Pharm Sci. 2023 Dec 1;191:106596. doi: 10.1016/j.ejps.2023.106596. Epub 2023 Sep 26. Eur J Pharm Sci. 2023. PMID: 37770004 Free PMC article. Review.
-
Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications.Gels. 2023 Mar 27;9(4):277. doi: 10.3390/gels9040277. Gels. 2023. PMID: 37102889 Free PMC article. Review.
-
Progress in Vocal Fold Regenerative Biomaterials: An Immunological Perspective.Adv Nanobiomed Res. 2022 Feb;2(2):2100119. doi: 10.1002/anbr.202100119. Epub 2021 Dec 18. Adv Nanobiomed Res. 2022. PMID: 35434718 Free PMC article.
-
Unraveling the Relevance of Tissue-Specific Decellularized Extracellular Matrix Hydrogels for Vocal Fold Regenerative Biomaterials: A Comprehensive Proteomic and In Vitro Study.Adv Nanobiomed Res. 2023 Apr;3(4):2200095. doi: 10.1002/anbr.202200095. Epub 2023 Jan 31. Adv Nanobiomed Res. 2023. PMID: 37547672 Free PMC article.
-
Material matters: exploring the interplay between natural biomaterials and host immune system.Front Immunol. 2023 Oct 23;14:1269960. doi: 10.3389/fimmu.2023.1269960. eCollection 2023. Front Immunol. 2023. PMID: 37936689 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

