Liquid Nitrogen-Based Cryoablation in In Vivo Porcine Tissue: A Pilot Study
- PMID: 33112569
- PMCID: PMC7798152
- DOI: 10.31557/APJCP.2020.21.10.3069
Liquid Nitrogen-Based Cryoablation in In Vivo Porcine Tissue: A Pilot Study
Abstract
Introduction: Liquid nitrogen-based cryoablation induces freezing evenly throughout the probe tip surface, resulting in larger ablation volumes and faster treatment times. The purpose of this preliminary investigation is to determine the efficacy of the liquid nitrogen-based Visica2 Cryoablation System (Sanarus Technologies, Pleasanton, CA) in in vivo porcine kidney, liver, and fibro-fatty tissue.
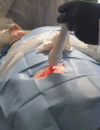
Methods: Ablations were performed under ultrasound guidance in 4 Yorkshire pigs. The target lesion cross-section width (W) and depth (D) were 1 cm for liver (n=8), kidney (n=4), and head-neck (n=5) and 2 cm for kidney (n=4). Expected axial length (L) of the resulting lesion is approximately 4 cm. After three-day survival, the ablated tissue was harvested and histologically analysed. The mean width and depth were compared with the target diameter using a one-sample t-test.
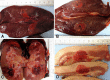
Results: All animals survived the procedure. For the 1 cm target, mean dimensions (L x W x D) were 3.8±1.5 x 1.7±0.3 x 1.7±0.7 for liver, 3.0±0.5 x 2.0±0.4 x 1.7±0.6 for kidney, and 3.3±0.8 x 1.8±0.4 x 1.8±0.4 for head-neck. Mean width and depth were significantly greater than desired dimension. For the 2 cm target, mean dimensions were 3.2±0.5 x 3.1±0.8 x 1.9±0.7. Mean width and depth were not significantly different to desired target.
Conclusion: Our preliminary results show that the Visica2 liquid nitrogen-based cryoablation system can efficiently and reproducibly create ablation volumes in liver, kidney, and fibro-fatty tissue within 4 minutes and 12 minutes for 1cm and 2cm targeted diameters, respectively. Further investigation is necessary to determine the optimal freeze-thaw-freeze protocol for larger ablation volumes.<br />.
Keywords: Cryotherapy; Liver cancer; Minimal invasive surgery; cryoablation; kidney cancer.
Figures



References
-
- Bahn DK, Lee F, Badalament R, et al. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60:3–11. - PubMed
-
- Edwards MJ, Broadwater R, Tafra L, et al. Progressive adoption of cryoablative therapy for breast fibroadenoma in community practice. Am J Surg. 2004;188:221–4. - PubMed
-
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. - PubMed

