Is Less More? Influence of the Coordination Geometry of Copper(II) Picolinate Chelate Complexes on Metabolic Stability
- PMID: 33112609
- PMCID: PMC9199361
- DOI: 10.1021/acs.inorgchem.0c02314
Is Less More? Influence of the Coordination Geometry of Copper(II) Picolinate Chelate Complexes on Metabolic Stability
Abstract
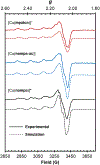
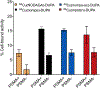
A growing number of copper(II) complexes have been identified as suitable candidates for biomedical applications. Here, we show that the biocompatibility and stability of copper(II) complexes can be tuned by directed ligand design and complex geometry. We demonstrate that azamacrocycle-based chelators that envelope copper(II) in a five-coordinate, distorted trigonal-bipyramidal structure are more chemically inert to redox-mediated structural changes than their six-coordinate, Jahn-Teller-distorted counterparts, as evidenced by electrochemical, crystallographic, electron paramagnetic resonance, and density functional theory studies. We further validated our hypothesis of enhanced inertness in vitro and in vivo by employing Cu-64 radiolabeling of bifunctional analogues appended to a prostate-specific membrane antigen targeting dipeptide. The corresponding Cu-64 complexes were tested for stability in vitro and in vivo, with the five-coordinate system demonstrating the greatest metabolic stability among the studied picolinate complex series.
Figures

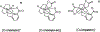



Similar articles
-
Monopicolinate cross-bridged cyclam combining very fast complexation with very high stability and inertness of its copper(II) complex.Inorg Chem. 2014 May 19;53(10):5269-79. doi: 10.1021/ic500491c. Epub 2014 Apr 23. Inorg Chem. 2014. PMID: 24758339
-
Copper(II) complexes of 1,10-phenanthroline-derived ligands: studies on DNA binding properties and nuclease activity.J Inorg Biochem. 2005 May;99(5):1205-19. doi: 10.1016/j.jinorgbio.2005.02.020. J Inorg Biochem. 2005. PMID: 15833344
-
Five coordinate M(II)-diphenolate [M = Zn(II), Ni(II), and Cu(II)] Schiff base complexes exhibiting metal- and ligand-based redox chemistry.Inorg Chem. 2013 Jan 18;52(2):660-70. doi: 10.1021/ic301731w. Epub 2013 Jan 8. Inorg Chem. 2013. PMID: 23297765
-
Lights and shadows in the challenge of binding acyclovir, a synthetic purine-like nucleoside with antiviral activity, at an apical-distal coordination site in copper(II)-polyamine chelates.J Inorg Biochem. 2015 Jul;148:84-92. doi: 10.1016/j.jinorgbio.2015.03.006. Epub 2015 Mar 23. J Inorg Biochem. 2015. PMID: 25863571
-
Ultrasonic synthesis of Oct. trans-Br2Cu(N ∩ N)2 Jahn-Teller distortion complex: XRD-properties, solvatochromism, thermal, kinetic and DNA-binding evaluations.Ultrason Sonochem. 2019 Apr;52:428-436. doi: 10.1016/j.ultsonch.2018.12.019. Epub 2018 Dec 12. Ultrason Sonochem. 2019. PMID: 30573435 Review.
Cited by
-
Coordination Chemistry of Sulfur-Containing Bifunctional Chelators: Toward in Vivo Stabilization of 64Cu PET Imaging Agents for Alzheimer's Disease.Inorg Chem. 2023 Dec 18;62(50):20820-20833. doi: 10.1021/acs.inorgchem.3c02929. Epub 2023 Dec 7. Inorg Chem. 2023. PMID: 38060375 Free PMC article.
-
An Overview of In Vitro Assays of 64Cu-, 68Ga-, 125I-, and 99mTc-Labelled Radiopharmaceuticals Using Radiometric Counters in the Era of Radiotheranostics.Diagnostics (Basel). 2023 Mar 23;13(7):1210. doi: 10.3390/diagnostics13071210. Diagnostics (Basel). 2023. PMID: 37046428 Free PMC article. Review.
-
Sensing a CO-Releasing Molecule (CORM) Does Not Equate to Sensing CO: The Case of DPHP and CORM-3.Anal Chem. 2023 Jun 13;95(23):9083-9089. doi: 10.1021/acs.analchem.3c01495. Epub 2023 Jun 1. Anal Chem. 2023. PMID: 37263968 Free PMC article.
-
Metal ion size profoundly affects H3glyox chelate chemistry.RSC Adv. 2021 Apr 27;11(26):15663-15674. doi: 10.1039/d1ra01793d. eCollection 2021 Apr 26. RSC Adv. 2021. PMID: 35481219 Free PMC article.
References
-
- Woodin KS; Heroux KJ; Boswell CA; Wong EH; Weisman GR; Niu W; Tomellini SA; Anderson CJ; Zakharov LN; Rheingold AL Kinetic Inertness and Electrochemical Behavior of Copper(II) Tetraazamacrocyclic Complexes: Possible Implications for in Vivo Stability. Eur. J. Inorg. Chem 2005, 2005, 4829–4833.
-
- Young MJ; Chin J Dinuclear Copper(II) Complex That Hydrolyzes RNA. J. Am. Chem. Soc 1995, 117, 10577–10578.
-
- Liu S The role of coordination chemistry in the development of target-specific radiopharmaceuticals. Chem. Soc. Rev 2004, 33 (7), 445–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

