Abdominal immune-related adverse events: detection on ultrasonography, CT, MRI and 18F-Fluorodeoxyglucose positron emission tomography
- PMID: 33112648
- PMCID: PMC7934307
- DOI: 10.1259/bjr.20200663
Abdominal immune-related adverse events: detection on ultrasonography, CT, MRI and 18F-Fluorodeoxyglucose positron emission tomography
Abstract
Immune checkpoint inhibitor and chimeric antigen receptor T-cell therapies are associated with a unique spectrum of complications termed immune-related adverse events (irAEs). The abdomen is the most frequent site of severe irAEs that require hospitalization with life-threatening consequences. Most abdominal irAEs such as enterocolitis, hepatitis, cholangiopathy, cholecystitis, pancreatitis, adrenalitis, and sarcoid-like reaction are initially detected on imaging such as ultrasonography (US), CT, MRI and fusion 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT during routine surveillance of cancer therapy. Early recognition and diagnosis of irAEs and immediate management with cessation of immune modulator cancer therapy and institution of immunosuppressive therapy are necessary to avert morbidity and mortality. Diagnosis of irAEs is confirmed by tissue sampling or by follow-up imaging demonstrating resolution. Abdominal radiologists reviewing imaging on patients being treated with anti-cancer immunomodulators should be familiar with the imaging manifestations of irAEs.
Figures

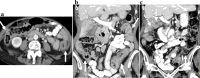
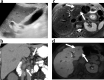
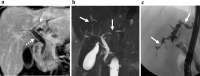
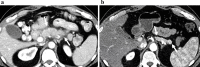
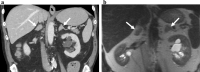
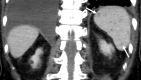
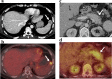
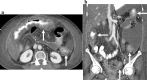



Similar articles
-
Frequency and imaging features of abdominal immune-related adverse events in metastatic lung cancer patients treated with PD-1 inhibitor.Abdom Radiol (NY). 2019 May;44(5):1917-1927. doi: 10.1007/s00261-019-01935-2. Abdom Radiol (NY). 2019. PMID: 30790009
-
Diagnostic impact of 18F-FDG PET/CT imaging on the detection of immune-related adverse events in patients treated with immunotherapy.Clin Transl Oncol. 2022 Oct;24(10):1903-1913. doi: 10.1007/s12094-022-02840-9. Epub 2022 May 20. Clin Transl Oncol. 2022. PMID: 35594002
-
Immunotherapy-related adverse effects on 18F-FDG PET/CT imaging.Br J Radiol. 2020 Jul;93(1111):20190832. doi: 10.1259/bjr.20190832. Epub 2020 Feb 27. Br J Radiol. 2020. PMID: 32105505 Free PMC article.
-
Imaging assessment of toxicity related to immune checkpoint inhibitors.Front Immunol. 2023 Feb 23;14:1133207. doi: 10.3389/fimmu.2023.1133207. eCollection 2023. Front Immunol. 2023. PMID: 36911692 Free PMC article. Review.
-
18F-FDG PET/CT and whole-body MRI diagnostic performance in M staging for non-small cell lung cancer: a systematic review and meta-analysis.Eur Radiol. 2020 Jul;30(7):3641-3649. doi: 10.1007/s00330-020-06703-1. Epub 2020 Mar 3. Eur Radiol. 2020. PMID: 32125513
Cited by
-
In-depth analysis of the safety of CAR-T cell therapy for solid tumors.Front Immunol. 2025 Feb 24;16:1548979. doi: 10.3389/fimmu.2025.1548979. eCollection 2025. Front Immunol. 2025. PMID: 40066440 Free PMC article. Review.
-
Is 18F-FDG-PET/CT an Optimal Imaging Modality for Detecting Immune-Related Adverse Events after Immune-Checkpoint Inhibitor Therapy? Pros and Cons.Cancers (Basel). 2024 May 24;16(11):1990. doi: 10.3390/cancers16111990. Cancers (Basel). 2024. PMID: 38893111 Free PMC article. Review.
-
Immunotherapy-related renal toxicity causes reversible renal enlargement.Abdom Radiol (NY). 2022 Sep;47(9):3301-3307. doi: 10.1007/s00261-022-03594-2. Epub 2022 Jul 1. Abdom Radiol (NY). 2022. PMID: 35776145
-
Acute Interstitial Nephritis on Positron-Emission Tomography-Computed Tomography Imaging.Kidney Med. 2022 Sep 30;4(11):100552. doi: 10.1016/j.xkme.2022.100552. eCollection 2022 Nov. Kidney Med. 2022. PMID: 36339667 Free PMC article. No abstract available.
-
Key CT and MRI findings of drug-associated hepatobiliary and pancreatic disorders.Jpn J Radiol. 2024 Mar;42(3):235-245. doi: 10.1007/s11604-023-01505-z. Epub 2023 Nov 6. Jpn J Radiol. 2024. PMID: 37926781 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

