Serine synthesis influences tamoxifen response in ER+ human breast carcinoma
- PMID: 33112838
- PMCID: PMC7780089
- DOI: 10.1530/ERC-19-0510
Serine synthesis influences tamoxifen response in ER+ human breast carcinoma
Abstract
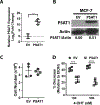
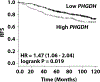
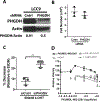
Estrogen receptor-positive breast cancer (ER+ BC) is the most common form of breast carcinoma accounting for approximately 70% of all diagnoses. Although ER-targeted therapies have improved survival outcomes for this BC subtype, a significant proportion of patients will ultimately develop resistance to these clinical interventions, resulting in disease recurrence. Phosphoserine aminotransferase 1 (PSAT1), an enzyme within the serine synthetic pathway (SSP), has been previously implicated in endocrine resistance. Therefore, we determined whether expression of SSP enzymes, PSAT1 or phosphoglycerate dehydrogenase (PHGDH), affects the response of ER+ BC to 4-hydroxytamoxifen (4-OHT) treatment. To investigate a clinical correlation between PSAT1, PHGDH, and endocrine resistance, we examined microarray data from ER+ patients who received tamoxifen as the sole endocrine therapy. We confirmed that higher PSAT1 and PHGDH expression correlates negatively with poorer outcomes in tamoxifen-treated ER+ BC patients. Next, we found that SSP enzyme expression and serine synthesis were elevated in tamoxifen-resistant compared to tamoxifen-sensitive ER+ BC cells in vitro. To determine relevance to endocrine sensitivity, we modified the expression of either PSAT1 or PHGDH in each cell type. Overexpression of PSAT1 in tamoxifen-sensitive MCF-7 cells diminished 4-OHT inhibition on cell proliferation. Conversely, silencing of either PSAT1 or PHGDH resulted in greater sensitivity to 4-OHT treatment in LCC9 tamoxifen-resistant cells. Likewise, the combination of a PHGDH inhibitor with 4-OHT decreased LCC9 cell proliferation. Collectively, these results suggest that overexpression of serine synthetic pathway enzymes contribute to tamoxifen resistance in ER+ BC, which can be targeted as a novel combinatorial treatment option.
Keywords: endocrine resistance; estrogen receptor-positive breast cancer; phosphoserine aminotransferase 1; serine synthesis; tamoxifen.
Conflict of interest statement
Conflict of Interest
All authors declare that there are no conflicts of interest.
Figures




References
-
- Andres SA & Wittliff JL 2012. Co-expression of genes with estrogen receptor-alpha and progesterone receptor in human breast carcinoma tissue. Horm Mol Biol Clin Investig, 12, 377–90. - PubMed
-
- Astruc ME, Chabret C, Bali P, Gagne D & Pons M 1995. Prolonged treatment of breast cancer cells with antiestrogens increases the activating protein-1-mediated response: involvement of the estrogen receptor. Endocrinology, 136, 824–32. - PubMed
-
- Bai Z & Gust R 2009. Breast cancer, estrogen receptor and ligands. Arch Pharm (Weinheim), 342, 133–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

