Fabrication routes via projection stereolithography for 3D-printing of microfluidic geometries for nucleic acid amplification
- PMID: 33112867
- PMCID: PMC7592796
- DOI: 10.1371/journal.pone.0240237
Fabrication routes via projection stereolithography for 3D-printing of microfluidic geometries for nucleic acid amplification
Abstract
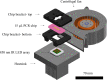
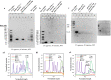
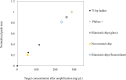
Digital Light Processing (DLP) stereolithography (SLA) as a high-resolution 3D printing process offers a low-cost alternative for prototyping of microfluidic geometries, compared to traditional clean-room and workshop-based methods. Here, we investigate DLP-SLA printing performance for the production of micro-chamber chip geometries suitable for Polymerase Chain Reaction (PCR), a key process in molecular diagnostics to amplify nucleic acid sequences. A DLP-SLA fabrication protocol for printed micro-chamber devices with monolithic micro-channels is developed and evaluated. Printed devices were post-processed with ultraviolet (UV) light and solvent baths to reduce PCR inhibiting residuals and further treated with silane coupling agents to passivate the surface, thereby limiting biomolecular adsorption occurences during the reaction. The printed devices were evaluated on a purpose-built infrared (IR) mediated PCR thermocycler. Amplification of 75 base pair long target sequences from genomic DNA templates on fluorosilane and glass modified chips produced amplicons consistent with the control reactions, unlike the non-silanized chips that produced faint or no amplicon. The results indicated good functionality of the IR thermocycler and good PCR compatibility of the printed and silanized SLA polymer. Based on the proposed methods, various microfluidic designs and ideas can be validated in-house at negligible costs without the requirement of tool manufacturing and workshop or clean-room access. Additionally, the versatile chemistry of 3D printing resins enables customised surface properties adding significant value to the printed prototypes. Considering the low setup and unit cost, design flexibility and flexible resin chemistries, DLP-SLA is anticipated to play a key role in future prototyping of microfluidics, particularly in the fields of research biology and molecular diagnostics. From a system point-of-view, the proposed method of thermocycling shows promise for portability and modular integration of funcitonalitites for diagnostic or research applications that utilize nucleic acid amplification technology.
Conflict of interest statement
The authors have read the journal’s policy and have the following potential competing interests: QuantumDx had a role in the study design. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures

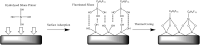




References
-
- Northup M., Ching M., White R. and Watson R., “DNA amplification with a microfabricated reaction chamber.,” 1993.
-
- Wilding P., Schoffner M. and Kricka L., “PCR in a silicon microstructure,” Clinical Chemistry, vol. 40, no. 9, pp. 1815–8, 1994. - PubMed
-
- Münchow G. and Drese K., “Chapter 13: Nucleic acid amplification in Microsystems,” in Microfluidic Technologies for Miniaturized Analysis Systems, Hardt S., Ed., Boston, MA, Springer, 2007, pp. 523–567. 10.1039/b612669n - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

