Development of a core outcome set for use in community-based bipolar trials-A qualitative study and modified Delphi
- PMID: 33112874
- PMCID: PMC7592842
- DOI: 10.1371/journal.pone.0240518
Development of a core outcome set for use in community-based bipolar trials-A qualitative study and modified Delphi
Abstract
Background: A core outcome set (COS) is a standardised collection of outcomes to be collected and reported in all trials within a research area. A COS can reduce reporting bias and facilitate evidence synthesis. This is currently unavailable for use in community-based bipolar trials. This research aimed to develop such a COS, with input from a full range of stakeholders.
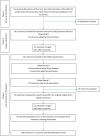
Methods: A co-production approach was used throughout. A longlist of outcomes was derived from focus groups with people with a bipolar diagnosis and carers, interviews with healthcare professionals and a rapid review of outcomes listed in bipolar trials on the Cochrane database. An expert panel with personal and/or professional experience of bipolar participated in a modified Delphi process and the COS was finalised at a consensus meeting.
Results: Fifty participants rated the importance of each outcome. Sixty-six outcomes were included in Round 1 of the questionnaire; 13 outcomes were added by Round 1 participants and were rated in Round 2. Seventy-six percent of participants (n = 38) returned to Round 2 and 60 outcomes, including 4 outcomes added by participants in Round 1, received a rating of 7-9 by >70% and 1-3 by <25% of the sample. Fourteen participants finalised a COS containing 11 outcomes at the consensus meeting: personal recovery; connectedness; clinical recovery of bipolar symptoms; mental health and wellbeing; physical health; self-monitoring and management; medication effects; quality of life; service outcomes; experience of care; and use of coercion.
Conclusions: This COS is recommended for use in community-based bipolar trials to ensure stakeholder-relevant outcomes, facilitate data synthesis, and transparent reporting. The COS includes guidance notes for each outcome to allow the identification of suitable measurement instruments. Further validation is recommended for use with a wide range of communities and to achieve standardised measurement.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: VP is the research director at McPin Foundation, a registered charity (1117336). She is a board member of the MRC developing mind programme, Rethink Mental Illness clinical advisory group, THIS Institute (Cambridge University) involvement and engagement panel. McPin receives funding from a variety of sources including NIHR / UKRI research grants, evaluation commissions from other charities and universities. MC receives funding from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, the NIHR Surgical Reconstruction and Microbiology Research Centre at the University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UK Research and Innovation), Macmillan Cancer Support, UCB Pharma and the Patient-Centered Outcomes Research Institute (PCORI). MC has received personal fees from Astellas, Daiichi Sankyo, Glaukos, Takeda, Merck, and Ferring outside the submitted work, and chairs the ISOQOL (International Society for Quality of Life Research) Best Practice for PROs in Trials Taskforce. TK is an employee of GlaxoSmithKline and completed work on this project while employed at Centre for Patient Reported Outcomes Research (CPROR), University of Birmingham (UoB). GT is currently funded by a Cancer Research UK Population Researcher Postdoctoral Fellowship award (reference: C56067/A21330). This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures
Similar articles
-
Development of a Core Outcome Set for Clinical Trials in Non-infectious Uveitis of the Posterior Segment.Ophthalmology. 2021 Aug;128(8):1209-1221. doi: 10.1016/j.ophtha.2021.01.022. Epub 2021 Jan 28. Ophthalmology. 2021. PMID: 33515595
-
Core outcome sets for use in effectiveness trials involving people with bipolar and schizophrenia in a community-based setting (PARTNERS2): study protocol for the development of two core outcome sets.Trials. 2015 Feb 12;16:47. doi: 10.1186/s13063-015-0553-0. Trials. 2015. PMID: 25887033 Free PMC article. Clinical Trial.
-
Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a 'core outcome set'.Trials. 2014 Jun 25;15:247. doi: 10.1186/1745-6215-15-247. Trials. 2014. PMID: 24962012 Free PMC article. Review.
-
A core outcomes set for clinical trials of interventions for young adults with type 1 diabetes: an international, multi-perspective Delphi consensus study.Trials. 2017 Dec 19;18(1):602. doi: 10.1186/s13063-017-2364-y. Trials. 2017. PMID: 29258565 Free PMC article.
-
COS-STAR: a reporting guideline for studies developing core outcome sets (protocol).Trials. 2015 Aug 22;16:373. doi: 10.1186/s13063-015-0913-9. Trials. 2015. PMID: 26297658 Free PMC article.
Cited by
-
Evaluation of randomized controlled trials: a primer and tutorial for mental health researchers.Trials. 2023 Aug 30;24(1):562. doi: 10.1186/s13063-023-07596-3. Trials. 2023. PMID: 37649083 Free PMC article.
-
Exploring the Personal Recovery Construct in Bipolar Disorders: Definition, Usage and Measurement. A Systematic Review.Front Psychiatry. 2022 Jun 23;13:876761. doi: 10.3389/fpsyt.2022.876761. eCollection 2022. Front Psychiatry. 2022. PMID: 35815013 Free PMC article.
-
Collaborative care approaches for people with severe mental illness.Cochrane Database Syst Rev. 2024 May 7;5(5):CD009531. doi: 10.1002/14651858.CD009531.pub3. Cochrane Database Syst Rev. 2024. PMID: 38712709 Free PMC article. Review.
-
Evaluation of a primary care-based collaborative care model (PARTNERS2) for people with diagnoses of schizophrenia, bipolar, or other psychoses: study protocol for a cluster randomised controlled trial.BJGP Open. 2021 Jun 30;5(3):BJGPO.2021.0033. doi: 10.3399/BJGPO.2021.0033. Print 2021 Jun. BJGP Open. 2021. PMID: 33785568 Free PMC article.
-
Synthesizing Core Outcome Sets for outcomes research in cohort studies: a systematic review.Pediatr Res. 2022 Oct;92(4):936-945. doi: 10.1038/s41390-021-01801-2. Epub 2021 Dec 17. Pediatr Res. 2022. PMID: 34921214 Free PMC article.
References
-
- Keeley T, Khan H, Pinfold V, Williamson P, Mathers J, Davies L, et al. Core outcome sets for use in effectiveness trials involving people with bipolar and schizophrenia in a community-based setting (PARTNERS2): study protocol for the development of two core outcome sets. Trials. 2015; 16:47 10.1186/s13063-015-0553-0 - DOI - PMC - PubMed
-
- World Health Organisation: International Classification of Diseases– 10 (Version: 2016)–Bipolar Affective Disorder. https://icd.who.int/browse10/2016/en#/F31 [Accessed 19.11.19].
-
- World Health Organisation: WHO releases new International Classification of Diseases (ICD 11). https://www.who.int/news-room/detail/18-06-2018-who-releases-new-interna... [Accessed 19.11.19].