Bioinformatic characterization of angiotensin-converting enzyme 2, the entry receptor for SARS-CoV-2
- PMID: 33112891
- PMCID: PMC7592753
- DOI: 10.1371/journal.pone.0240647
Bioinformatic characterization of angiotensin-converting enzyme 2, the entry receptor for SARS-CoV-2
Abstract
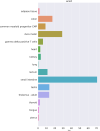
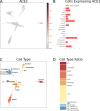
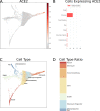
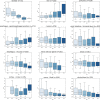
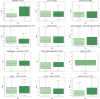
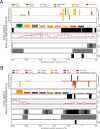
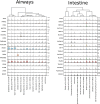
The World Health Organization declared the COVID-19 epidemic a public health emergency of international concern on March 11th, 2020, and the pandemic is rapidly spreading worldwide. COVID-19 is caused by a novel coronavirus SARS-CoV-2, which enters human target cells via angiotensin converting enzyme 2 (ACE2). We used a number of bioinformatics tools to computationally characterize ACE2 by determining its cell-specific expression in trachea, lung, and small intestine, derive its putative functions, and predict transcriptional regulation. The small intestine expressed higher levels of ACE2 mRNA than any other organ. By immunohistochemistry, duodenum, kidney and testis showed strong signals, whereas the signal was weak in the respiratory tract. Single cell RNA-Seq data from trachea indicated positive signals along the respiratory tract in key protective cell types including club, goblet, proliferating, and ciliary epithelial cells; while in lung the ratio of ACE2-expressing cells was low in all cell types (<2.6%), but was highest in vascular endothelial and goblet cells. Gene ontology analysis suggested that, besides its classical role in the renin-angiotensin system, ACE2 may be functionally associated with angiogenesis/blood vessel morphogenesis. Using a novel tool for the prediction of transcription factor binding sites we identified several putative binding sites within two tissue-specific promoters of the ACE2 gene as well as a new putative short form of ACE2. These include several interferon-stimulated response elements sites for STAT1, IRF8, and IRF9. Our results also confirmed that age and gender play no significant role in the regulation of ACE2 mRNA expression in the lung.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2.Circ Res. 2020 May 8;126(10):1456-1474. doi: 10.1161/CIRCRESAHA.120.317015. Epub 2020 Apr 8. Circ Res. 2020. PMID: 32264791 Free PMC article. Review.
-
ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities.Elife. 2020 Nov 9;9:e61390. doi: 10.7554/eLife.61390. Elife. 2020. PMID: 33164751 Free PMC article. Review.
-
SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy.Theranostics. 2020 Jun 12;10(16):7448-7464. doi: 10.7150/thno.48076. eCollection 2020. Theranostics. 2020. PMID: 32642005 Free PMC article. Review.
-
SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues.Cell. 2020 May 28;181(5):1016-1035.e19. doi: 10.1016/j.cell.2020.04.035. Epub 2020 Apr 27. Cell. 2020. PMID: 32413319 Free PMC article.
-
Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses.Biochem Biophys Res Commun. 2020 May 21;526(1):135-140. doi: 10.1016/j.bbrc.2020.03.044. Epub 2020 Mar 19. Biochem Biophys Res Commun. 2020. PMID: 32199615 Free PMC article.
Cited by
-
Intestinal Receptor of SARS-CoV-2 in Inflamed IBD Tissue Seems Downregulated by HNF4A in Ileum and Upregulated by Interferon Regulating Factors in Colon.J Crohns Colitis. 2021 Mar 5;15(3):485-498. doi: 10.1093/ecco-jcc/jjaa185. J Crohns Colitis. 2021. PMID: 32915959 Free PMC article.
-
Role of Extracellular Vesicles in Crohn's Patients on Adalimumab Who Received COVID-19 Vaccination.Int J Mol Sci. 2024 Aug 14;25(16):8853. doi: 10.3390/ijms25168853. Int J Mol Sci. 2024. PMID: 39201543 Free PMC article.
-
COVID-19 on Oral Health: A New Bilateral Connection for the Pandemic.Biomedicines. 2023 Dec 26;12(1):60. doi: 10.3390/biomedicines12010060. Biomedicines. 2023. PMID: 38255167 Free PMC article. Review.
-
An update on angiotensin-converting enzyme 2 structure/functions, polymorphism, and duplicitous nature in the pathophysiology of coronavirus disease 2019: Implications for vascular and coagulation disease associated with severe acute respiratory syndrome coronavirus infection.Front Microbiol. 2022 Nov 28;13:1042200. doi: 10.3389/fmicb.2022.1042200. eCollection 2022. Front Microbiol. 2022. PMID: 36519165 Free PMC article. Review.
-
Investigating the human protein-host protein interactome of SARS-CoV-2 infection in the small intestine.Gastroenterol Hepatol Bed Bench. 2020 Fall;13(4):374-387. Gastroenterol Hepatol Bed Bench. 2020. PMID: 33244381 Free PMC article.
References
-
- Meldrum NU, Roughton FJW. Some properties of carbonic anhydrase, the CO2 enzyme present in blood. J Physiol. 1932;75:15–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

