A new perfusion culture method with a self-organized capillary network
- PMID: 33112918
- PMCID: PMC7592787
- DOI: 10.1371/journal.pone.0240552
A new perfusion culture method with a self-organized capillary network
Abstract
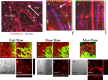
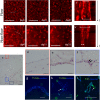
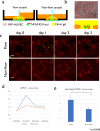
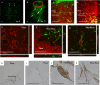
A lack of perfusion has been one of the most significant obstacles for three-dimensional culture systems of organoids and embryonic tissues. Here, we developed a simple and reliable method to implement a perfusable capillary network in vitro. The method employed the self-organization of endothelial cells to generate a capillary network and a static pressure difference for culture medium circulation, which can be easily introduced to standard biological laboratories and enables long-term cultivation of vascular structures. Using this culture system, we perfused the lumen of the self-organized capillary network and observed a flow-induced vascular remodeling process, cell shape changes, and collective cell migration. We also observed an increase in cell proliferation around the self-organized vasculature induced by flow, indicating functional perfusion of the culture medium. We also reconstructed extravasation of tumor and inflammatory cells, and circulation inside spheroids including endothelial cells and human lung fibroblasts. In conclusion, this system is a promising tool to elucidate the mechanisms of various biological processes related to vascular flow.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

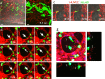

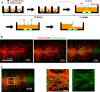
Similar articles
-
In vitrovascularization of hydrogel-based tissue constructs via a combined approach of cell sheet engineering and dynamic perfusion cell culture.Biofabrication. 2022 Oct 27;15(1). doi: 10.1088/1758-5090/ac9433. Biofabrication. 2022. PMID: 36300786
-
Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device.Integr Biol (Camb). 2017 Jun 19;9(6):506-518. doi: 10.1039/c7ib00024c. Integr Biol (Camb). 2017. PMID: 28561127
-
Engineering a Blood Vessel Network Module for Body-on-a-Chip Applications.J Lab Autom. 2015 Jun;20(3):296-301. doi: 10.1177/2211068214562831. Epub 2014 Dec 22. J Lab Autom. 2015. PMID: 25532526
-
Engineering next generation vascularized organoids.Atherosclerosis. 2024 Nov;398:118529. doi: 10.1016/j.atherosclerosis.2024.118529. Epub 2024 Jul 1. Atherosclerosis. 2024. PMID: 39304390 Review.
-
Technical and theoretical considerations about gradient perfusion culture for epithelia used in tissue engineering, biomaterial testing and pharmaceutical research.Biomed Mater. 2007 Jun;2(2):R1-R11. doi: 10.1088/1748-6041/2/2/R01. Epub 2007 Mar 7. Biomed Mater. 2007. PMID: 18458434 Review.
Cited by
-
Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases.Cells. 2021 Nov 15;10(11):3183. doi: 10.3390/cells10113183. Cells. 2021. PMID: 34831406 Free PMC article. Review.
-
The Vascular Microenvironment in Glioblastoma: A Comprehensive Review.Biomedicines. 2022 May 31;10(6):1285. doi: 10.3390/biomedicines10061285. Biomedicines. 2022. PMID: 35740307 Free PMC article. Review.
-
Engineering aortic valves via transdifferentiating fibroblasts into valvular endothelial cells without using viruses or iPS cells.Bioact Mater. 2024 Nov 23;45:181-200. doi: 10.1016/j.bioactmat.2024.11.018. eCollection 2025 Mar. Bioact Mater. 2024. PMID: 39651397 Free PMC article.
-
Improvement of the cell viability of hepatocytes cultured in three-dimensional collagen gels using pump-free perfusion driven by water level difference.Sci Rep. 2022 Nov 24;12(1):20269. doi: 10.1038/s41598-022-24423-y. Sci Rep. 2022. PMID: 36434099 Free PMC article.
-
Transforming Capillary Alginate Gel (Capgel) into New 3D-Printing Biomaterial Inks.Gels. 2022 Jun 14;8(6):376. doi: 10.3390/gels8060376. Gels. 2022. PMID: 35735720 Free PMC article.
References
-
- Gilbert SF, Michael J. Barresi. Developmental Biology. 11th ed. Sinauer; 2018.
-
- Wolpert L, Tickle C. Principles of development. 4th ed. New York, New York, USA: Oxford University Press; 2011.
-
- Sadler TW. Langmanʼs Medical Embryology. 10th ed. Baltimore: Lippincott Williams & Wilkins; 2006. 10.1097/00006534-198801000-00024 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

