Unexpected photosensitivity of the well-characterized heme enzyme chlorite dismutase
- PMID: 33113038
- PMCID: PMC7665973
- DOI: 10.1007/s00775-020-01826-8
Unexpected photosensitivity of the well-characterized heme enzyme chlorite dismutase
Abstract
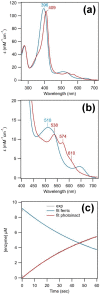
Chlorite dismutase is a heme enzyme that catalyzes the conversion of the toxic compound ClO2- (chlorite) to innocuous Cl- and O2. The reaction is a very rare case of enzymatic O-O bond formation, which has sparked the interest to elucidate the reaction mechanism using pre-steady-state kinetics. During stopped-flow experiments, spectroscopic and structural changes of the enzyme were observed in the absence of a substrate in the time range from milliseconds to minutes. These effects are a consequence of illumination with UV-visible light during the stopped-flow experiment. The changes in the UV-visible spectrum in the initial 200 s of the reaction indicate a possible involvement of a ferric superoxide/ferrous oxo or ferric hydroxide intermediate during the photochemical inactivation. Observed EPR spectral changes after 30 min reaction time indicate the loss of the heme and release of iron during the process. During prolonged illumination, the oligomeric state of the enzyme changes from homo-pentameric to monomeric with subsequent protein precipitation. Understanding the effects of UV-visible light illumination induced changes of chlorite dismutase will help us to understand the nature and mechanism of photosensitivity of heme enzymes in general. Furthermore, previously reported stopped-flow data of chlorite dismutase and potentially other heme enzymes will need to be re-evaluated in the context of the photosensitivity. Illumination of recombinantly expressed Azospira oryzae Chlorite dismutase (AoCld) with a high-intensity light source, common in stopped-flow equipment, results in disruption of the bond between FeIII and the axial histidine. This leads to the enzyme losing its heme cofactor and changing its oligomeric state as shown by spectroscopic changes and loss of activity.
Keywords: Azospira oryzae; Chlorite dismutase; Electron paramagnetic resonance; Heme enzyme; Oligomeric state; Photosensitivity; Stopped-flow spectroscopy; UV–visible illumination.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

