Depletion of LAG-3+ T Cells Translated to Pharmacology and Improvement in Psoriasis Disease Activity: A Phase I Randomized Study of mAb GSK2831781
- PMID: 33113155
- PMCID: PMC8246744
- DOI: 10.1002/cpt.2091
Depletion of LAG-3+ T Cells Translated to Pharmacology and Improvement in Psoriasis Disease Activity: A Phase I Randomized Study of mAb GSK2831781
Abstract
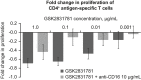
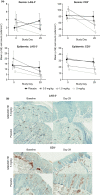
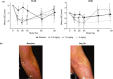
Activated T cells drive a range of immune-mediated inflammatory diseases. LAG-3 is transiently expressed on recently activated CD4+ and CD8+ T cells. We describe the engineering and first-in-human clinical study (NCT02195349) of GSK2831781 (an afucosylated humanized IgG1 monoclonal antibody enhanced with high affinity for Fc receptors and LAG-3 and antibody-dependent cellular cytotoxicity capabilities), which depletes LAG-3 expressing cells. GSK2831781 was tested in a phase I/Ib, double-blind, placebo-controlled clinical study, which randomized 40 healthy participants (part A) and 27 patients with psoriasis (part B) to single doses of GSK2831781 (up to 0.15 and 5 mg/kg, respectively) or placebo. Adverse events were generally balanced across groups, with no safety or tolerability concern identified. LAG-3+ cell depletion in peripheral blood was observed at doses ≥ 0.15 mg/kg and was dose-dependent. In biopsies of psoriasis plaques, a reduction in mean group LAG-3+ and CD3+ T-cell counts was observed following treatment. Downregulation of proinflammatory genes (IL-17A, IL-17F, IFNγ, and S100A12) and upregulation of the epithelial barrier integrity gene, CDHR1, was observed with the 5 mg/kg dose of GSK2831781. Psoriasis disease activity improved up to day 43 at all GSK2831781 doses (0.5, 1.5, and 5 mg/kg) compared with placebo. Depletion of LAG-3-expressing activated T cells is a novel approach, and this first clinical study shows that GSK2831781 is pharmacologically active and provides encouraging early evidence of clinical effects in psoriasis, which warrants further investigation in T-cell-mediated inflammatory diseases.
© 2020 GlaxoSmithKline. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.E., D.J.B.M., N.S., A.R., L.L., K.L., K.L.N., S.T., S.A.H., L.F., K.E., Y.C., R.A., M.F., T.M.W., S.J.B., N.W., and R.M.T. are employees of, and hold stocks/share options in, GSK. C.B., C.J.D., E.C., J.S., and C.O.S.S. were employees of GSK at the time of the study and hold stocks/share options in GSK. T.S.S. and T.G.H. were employees of GSK at the time of the study. R.F. and M.A. were employees of Parexel International at the time of the study; Parexel International was funded for the conduct of the study by GSK. There are patents and patent applications related to the development of GSK2831781 and its medical uses thereof. M.C. declared no competing interests for this work.
Figures


References
-
- Baker, K.F. & Isaacs, J.D. Novel therapies for immune‐mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann. Rheum. Dis. 77, 175–187 (2018). - PubMed
-
- Duijvestein, M. et al. Novel therapies and treatment strategies for patients with inflammatory bowel disease. Curr. Treat. Options Gastroenterol. 16, 129–146 (2018). - PubMed
-
- McKay, C. , Kondratuk, K.E. , Miller, J.P. , Stumpf, B. & Boh, E. Biologic therapy in psoriasis: navigating the options. Cutis 102, 13–17 (2018). - PubMed
-
- Alnaimat, F. , Sidhu, P. & Sarkar, S. T‐cell targeted therapies in autoimmune diseases. Drug Dev. Res. 72, 585–597 (2011).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

