Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients
- PMID: 33113551
- PMCID: PMC7746126
- DOI: 10.1182/blood.2020008824
Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients
Abstract
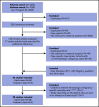
Outcomes for patients with hematologic malignancy infected with COVID-19 have not been aggregated. The objective of this study was to perform a systematic review and meta-analysis to estimate the risk of death and other important outcomes for these patients. We searched PubMed and EMBASE up to 20 August 2020 to identify reports of patients with hematologic malignancy and COVID-19. The primary outcome was a pooled mortality estimate, considering all patients and only hospitalized patients. Secondary outcomes included risk of intensive care unit admission and ventilation in hospitalized patients. Subgroup analyses included mortality stratified by age, treatment status, and malignancy subtype. Pooled prevalence, risk ratios (RRs), and 95% confidence intervals (CIs) were calculated using a random-effects model. Thirty-four adult and 5 pediatric studies (3377 patients) from Asia, Europe, and North America were included (14 of 34 adult studies included only hospitalized patients). Risk of death among adult patients was 34% (95% CI, 28-39; N = 3240) in this sample of predominantly hospitalized patients. Patients aged ≥60 years had a significantly higher risk of death than patients <60 years (RR, 1.82; 95% CI, 1.45-2.27; N = 1169). The risk of death in pediatric patients was 4% (95% CI, 1-9; N = 102). RR of death comparing patients with recent systemic anticancer therapy to no treatment was 1.17 (95% CI, 0.83-1.64; N = 736). Adult patients with hematologic malignancy and COVID-19, especially hospitalized patients, have a high risk of dying. Patients ≥60 years have significantly higher mortality; pediatric patients appear to be relatively spared. Recent cancer treatment does not appear to significantly increase the risk of death.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.C. has received research funding from Takeda, Celgene, Janssen, and IQVIA, and has provided consultancy for Takeda, Celgene, Janssen, Sanofi, Amgen, Roche, and Karyopharm. B.F. has received consultation fees from Momenta Pharmaceuticals and Apellis SRL on autoimmune hemolytic anemia. J.Z. has received research funding from Incyte and Quercegen; has provided consultancy for Sanofi, CSL, and Parexel; and has received honoraria or served on advisory boards for Pfizer/Bristol Myers Squibb, Portola, and Dova. L.S. has received honoraria from AbbVie, AstraZeneca, Gilead, and Janssen. W.A.W. has received research funding from Pfizer and Genentech, is a consultant for Best Doctors/Teladoc, is an advisor for and holds equity in Koneksa Health and Elektra Labs, and has received honoraria from the ASH Research Collaborative. L.K.H. is co–principal investigator on a study partially funded by Gilead Sciences. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Under COVID of the night.Blood. 2020 Dec 17;136(25):2844-2845. doi: 10.1182/blood.2020009377. Blood. 2020. PMID: 33331931 Free PMC article.
-
A rationale to prioritise vaccination of HSCT patients against COVID-19.Lancet Haematol. 2021 Mar;8(3):e163-e164. doi: 10.1016/S2352-3026(21)00008-9. Epub 2021 Jan 19. Lancet Haematol. 2021. PMID: 33482112 Free PMC article. No abstract available.
References
-
- Brissot E, Labopin M, Baron F, et al. Management of patients with acute leukemia during the COVID-19 outbreak: practical guidelines from the acute leukemia working party of the European Society for Blood and Marrow Transplantation [published online ahead of print 11 June 2020]. Bone Marrow Transplant. doi:10.1038/s41409-020-0970-x. - PMC - PubMed
-
- Ljungman P, Mikulska M, de la Camara R, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy [published correction appears in Bone Marrow Transplant. doi:10.1038/s41409-020-0965-7, published online ahead of print 8 June 2020]. Bone Marrow Transplant. 2020;55(11):2071-2076. - PMC - PubMed
-
- ESMO Cancer patient management during the COVID-19 pandemic. https://www.esmo.org/guidelines/cancer-patient-management-during-the-cov.... Accessed 1 September 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

