Roles of Farnesyl-Diphosphate Farnesyltransferase 1 in Tumour and Tumour Microenvironments
- PMID: 33113804
- PMCID: PMC7693003
- DOI: 10.3390/cells9112352
Roles of Farnesyl-Diphosphate Farnesyltransferase 1 in Tumour and Tumour Microenvironments
Abstract
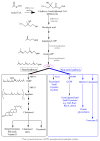
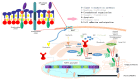
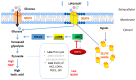
Farnesyl-diphosphate farnesyltransferase 1 (FDFT1, squalene synthase), a membrane-associated enzyme, synthesizes squalene via condensation of two molecules of farnesyl pyrophosphate. Accumulating evidence has noted that FDFT1 plays a critical role in cancer, particularly in metabolic reprogramming, cell proliferation, and invasion. Based on these advances in our knowledge, FDFT1 could be a potential target for cancer treatment. This review focuses on the contribution of FDFT1 to the hallmarks of cancer, and further, we discuss the applicability of FDFT1 as a cancer prognostic marker and target for anticancer therapy.
Keywords: cholesterol synthesis; farnesyl-diphosphate farnesyltransferase 1; lipid rafts; prognostic marker; tumour progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

