Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors
- PMID: 33113857
- PMCID: PMC7692789
- DOI: 10.3390/jcm9113417
Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors
Abstract
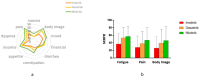
The life expectancy of patients with chronic myeloid leukemia (CML) approaches that of the age-matched population and quality of life (QOL) issues are becoming increasingly important. To describe patients' characteristics and assess QOL, we delivered a 30-item core questionnaire, a 24-item CML-specific questionnaire, both from the European Organization for Research and Treatment of Cancer (EORTC), and additional health-related items to 350 patients. Among 193 patients who completed the questionnaires, 139 received either imatinib (n = 70, 33%), dasatinib (n = 45, 23%) or nilotinib (n = 24, 12%). Patients' median age was 58 (range: 23 to 89) years and 86 (63%) were males. Stratifying patients by treatment, we recognized two distinct populations. In comparison to patients on dasatinib and nilotinib, patients on imatinib were two decades older, had a longer duration of disease and current treatment, experienced fewer limitations on daily activities (p = 0.02), less fatigue (p = 0.001), lower degree of impaired body image (p = 0.022) and less painful episodes (p = 0.014). Similarly, they had better emotional functioning, were less worried, stressed, depressed or nervous (p = 0.01) and were more satisfied with their treatment (p = 0.018). Not only does age associate with current treatments, but it also predicts how patients perceive QOL. Young patients express impaired QOL compared with elderly patients.
Keywords: chronic myeloid leukemia; patient-reported outcome; quality of life; tyrosine kinase inhibitors.
Conflict of interest statement
Adi Shacham Abulafia—participated in advisory boards of Novartis, Pfizer and BMS. Pia Raanani—participated in advisory boards of Novartis, Pfizer, BMS and Incyte. Giora Sharf—participated in advisory boards of Novartis, Pfizer, BMS, Medisson, Takeda and Incyte. Uri Rozovski, Avi Leader, Sivan Shemesh, Tamar Bergerand Lena Rosenmann declare no conflict of interest.
Figures
References
-
- Cohen M.H., Johnson J.R., Pazdur R. U.S. Food and Drug Administration Drug Approval Summary: Conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin. Cancer Res. 2005;11:12–19. - PubMed
-
- Hoffmann V.S., Baccarani M., Hasford J., Castagnetti F., Di Raimondo F., Casado L.F., Turkina A., Zackova D., Ossenkoppele G., Zaritskey A., et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia. 2016;31:593–601. doi: 10.1038/leu.2016.246. - DOI - PubMed
-
- Sasaki K., Strom S.S., O’Brien S., Jabbour E., Ravandi F., Konopleva M., Borthakur G., Pemmaraju N., Daver N., Jain P., et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: Analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–e193. doi: 10.1016/S2352-3026(15)00048-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

