Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids
- PMID: 33113950
- PMCID: PMC7660210
- DOI: 10.3390/molecules25214901
Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids
Abstract
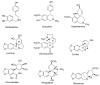
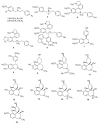
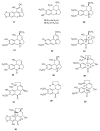
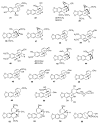
Alkaloids are an important group of specialized nitrogen metabolites with a wide range of biochemical and pharmacological effects. Since the first publication on lycorine in 1877, more than 650 alkaloids have been extracted from Amaryllidaceae bulbous plants and clustered together as the Amaryllidaceae alkaloids (AAs) family. AAs are specifically remarkable for their diverse pharmaceutical properties, as exemplified by the success of galantamine used to treat the symptoms of Alzheimer's disease. This review addresses the isolation, biological, and structure activity of AAs discovered from January 2015 to August 2020, supporting their therapeutic interest.
Keywords: Amaryllidaceae alkaloids; anti-cholinesterase; antiparasitic; antitumor; antiviral; biosynthesis; specialized metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

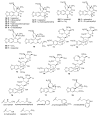
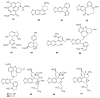
References
-
- Singh A., Desgagné-Penix I. Biosynthesis of the Amaryllidaceae alkaloids. Plant. Sci. Today. 2014;1:114–120. doi: 10.14719/pst.2014.1.3.41. - DOI
-
- Gerrard A.W. The proximate principles of the Narcissus pseudonarcissus. Pharm. J. 1877;8:214.
-
- Asahina Y., Sugii Y. Ueber die Identitaet des Lycorins und Narcissins. Arch. Pharm. 1913;251:357. doi: 10.1002/ardp.19132510507. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

