Targeting P53 as a Future Strategy to Overcome Gemcitabine Resistance in Biliary Tract Cancers
- PMID: 33113997
- PMCID: PMC7690712
- DOI: 10.3390/biom10111474
Targeting P53 as a Future Strategy to Overcome Gemcitabine Resistance in Biliary Tract Cancers
Abstract
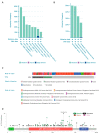
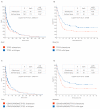
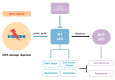
Gemcitabine-based chemotherapy is the current standard treatment for biliary tract cancers (BTCs) and resistance to gemcitabine remains the clinical challenge. TP53 mutation has been shown to be associated with poor clinicopathologic characteristics and survival in patients with BTCs, indicating that p53 plays an important role in the treatment of these cancers. Herein, we comprehensively reviewed previous BTC preclinical research and early clinical trials in terms of p53, as well as novel p53-targeted treatment, alone or in combination with either chemotherapy or other targeted therapies in BTCs. Preclinical studies have demonstrated that p53 mutations in BTCs are associated with enhanced gemcitabine resistance, therefore targeting p53 may be a novel therapeutic strategy for treatment of BTCs. Directly targeting mutant p53 by p53 activators, or indirectly by targeting cell cycle checkpoint proteins (Chk1, ataxia telangiectasia related (ATR), and Wee1) leading to synthetic lethality, may be potential future strategies for gemcitabine-resistant p53 mutated BTCs. In contrast, for wild-type p53 BTCs, activation of p53 by inhibition of its negative regulators (MDM2 and wild-type p53-induced phosphatase 1 (WIP1)) may be alternative options. Combination therapies consisting of standard cytotoxic drugs and novel small molecules targeting p53 and related signaling pathways may be the future key standard approach to beat cancer.
Keywords: biliary tract cancer; gemcitabine resistance; p53.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas.BMC Cancer. 2010 Nov 18;10:631. doi: 10.1186/1471-2407-10-631. BMC Cancer. 2010. PMID: 21087480 Free PMC article.
-
Effect of midkine on gemcitabine resistance in biliary tract cancer.Int J Mol Med. 2018 Apr;41(4):2003-2011. doi: 10.3892/ijmm.2018.3399. Epub 2018 Jan 18. Int J Mol Med. 2018. PMID: 29344648 Free PMC article.
-
Modulation of Biliary Cancer Chemo-Resistance Through MicroRNA-Mediated Rewiring of the Expansion of CD133+ Cells.Hepatology. 2020 Sep;72(3):982-996. doi: 10.1002/hep.31094. Epub 2020 Sep 10. Hepatology. 2020. PMID: 31879968 Free PMC article.
-
First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: "And Yet It Moves!".Cancer Treat Res Commun. 2021;27:100335. doi: 10.1016/j.ctarc.2021.100335. Epub 2021 Feb 11. Cancer Treat Res Commun. 2021. PMID: 33592561 Review.
-
MDM2 as a therapeutic target in advanced biliary tract cancers.Oncologist. 2025 May 8;30(5):oyaf094. doi: 10.1093/oncolo/oyaf094. Oncologist. 2025. PMID: 40421959 Free PMC article. Review.
Cited by
-
Prioritizing key synergistic circulating microRNAs for the early diagnosis of biliary tract cancer.Front Oncol. 2022 Oct 6;12:968412. doi: 10.3389/fonc.2022.968412. eCollection 2022. Front Oncol. 2022. PMID: 36276146 Free PMC article.
-
Precision Medicine in Cholangiocarcinoma: Past, Present, and Future.Life (Basel). 2022 Jun 2;12(6):829. doi: 10.3390/life12060829. Life (Basel). 2022. PMID: 35743860 Free PMC article. Review.
-
Induction of DR5-Dependent Apoptosis by PGA2 through ATF4-CHOP Pathway.Molecules. 2022 Jun 13;27(12):3804. doi: 10.3390/molecules27123804. Molecules. 2022. PMID: 35744931 Free PMC article.
-
Synergistic Inhibition of Pancreatic Cancer Cell Growth and Migration by Gemcitabine and Withaferin A.Biomolecules. 2024 Sep 19;14(9):1178. doi: 10.3390/biom14091178. Biomolecules. 2024. PMID: 39334944 Free PMC article.
-
Chinese expert consensus on the clinical application of molecular diagnostics in hepatobiliary cancers (2024 edition).Liver Res. 2024 Dec 3;8(4):195-206. doi: 10.1016/j.livres.2024.11.005. eCollection 2024 Dec. Liver Res. 2024. PMID: 39958921 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

