Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels
- PMID: 33114095
- PMCID: PMC7690814
- DOI: 10.3390/plants9111419
Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels
Abstract
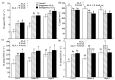
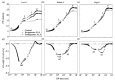
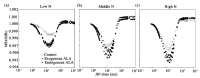
Limited data are available on the effects of 5-aminolevulinic acid (ALA) on plant photosynthesis in relation to the nitrogen (N) level. In this study, we investigate photosynthetic responses to ALA in canola plants (Brassica napus L.). We used wild-type plants without ALA addition (controls), wild-type plants with exogenous ALA application, and transgenic plants that endogenously overproduced ALA. The plants were grown hydroponically in nutrient solutions with low, middle, and high concentrations of N. Our results indicate that plants in both treatment groups had higher chlorophyll contents and net photosynthetic rates and lower intracellular CO2 concentrations in the leaves, as compared to controls. Furthermore, simultaneous measurement of prompt chlorophyll fluorescence and modulated 820-nm reflections showed that the active photosystem II (PS II) reaction centers, electron transfer capacity, and photosystem I (PS I) activity were all higher in treated plants than controls at all N levels; however, the responses of some photochemical processes to ALA were significantly affected by the N level. For example, under low N conditions only, a negative ΔK peak appeared in the prompt chlorophyll fluorescence curve, indicating a protective effect of ALA on electron donation via activation of the oxygen-evolving complex. Taken together, our findings suggest that ALA contributes to the promotion of photosynthesis by regulating photosynthetic electron transport under various N levels. These findings may provide a new strategy for improving photosynthesis in crops grown in N-poor conditions or reduced N-fertilization requirements.
Keywords: 5-aminolevulinic acid (ALA); canola; nitrogen supply; photosynthetic responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Scharf P.C., Wiebold W.J., Lory J.A. Corn yield response to nitrogen fertilizer timing and deficiency level. Agron. J. 2002;94:435–441. doi: 10.2134/agronj2002.4350. - DOI
-
- Gong X.W., Li J., Ma H.C., Chen G.H., Dang K., Yang P., Wang M., Feng B.L. Nitrogen deficiency induced a decrease in grain yield related to photosynthetic characteristics, carbon–nitrogen balance and nitrogen use efficiency in proso millet (Panicum miliaceum L.) Arch. Agron. Soil Sci. 2020;66:398–413. doi: 10.1080/03650340.2019.1619077. - DOI
Grants and funding
- 31772253/the National Natural Science Foundation of China
- KYYJ202004/the Fundamental Research Funds for the Central Universities
- no/the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions
- SXYBKY201736/the Excellent PhD Reward Project Fund of Shanxi Province
LinkOut - more resources
Full Text Sources

