Identification of a Reliable Biomarker Profile for the Diagnosis of Gaucher Disease Type 1 Patients Using a Mass Spectrometry-Based Metabolomic Approach
- PMID: 33114153
- PMCID: PMC7660648
- DOI: 10.3390/ijms21217869
Identification of a Reliable Biomarker Profile for the Diagnosis of Gaucher Disease Type 1 Patients Using a Mass Spectrometry-Based Metabolomic Approach
Abstract
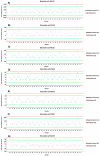
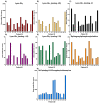
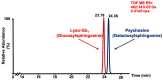
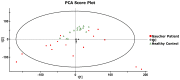
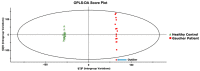
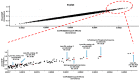
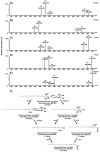
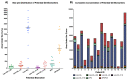
Gaucher disease (GD) is a rare autosomal recessive multisystemic lysosomal storage disorder presenting a marked phenotypic and genotypic variability. GD is caused by a deficiency in the glucocerebrosidase enzyme. The diagnosis of GD remains challenging because of the large clinical spectrum associated with the disease. Moreover, GD biomarkers are often not sensitive enough and can be subject to polymorphic variations. The main objective of this study was to perform a metabolomic study using an ultra-performance liquid chromatography system coupled to a time-of-flight mass spectrometer to identify novel GD biomarkers. Following the analysis of plasma samples from patients with GD, and age- and gender-matched control samples, supervised statistical analyses were used to find the best molecules to differentiate the two groups. Targeted biomarkers were structurally elucidated using accurate mass measurements and tandem mass spectrometry. This metabolomic study was successful in highlighting seven biomarkers associated with GD. Fragmentation tests revealed that these latter biomarkers were lyso-Gb1 (glucosylsphingosine) and four related analogs (with the following modifications on the sphingosine moiety: -C2H4, -H2, -H2+O, and +H2O), sphingosylphosphorylcholine, and N-palmitoyl-O-phosphocholineserine. Based on the plasma biomarker distribution, we suggest the evaluation of this GD biomarker profile, which might facilitate early diagnosis, monitoring, and follow-up of patients.
Keywords: Gaucher disease; N-palmitoyl-O-phosphocholineserine; biomarkers; glucosylsphingosine (lyso-Gb1); lyso-Gb1 analogs; mass spectrometry; metabolomics; plasma; sphingosylphosphorylcholine.
Conflict of interest statement
F.E.M. was paid for an advisory board by Sanofi-Genzyme. A.A. and G.-É.R. declare that they have no conflict of interest. I.M. received a student stipend from Shire/Takeda Pharmaceutical for this project. M.B. received a salary. C.A.-B. received research grants and honoraria from Shire/Takeda Pharmaceutical.
Figures


References
-
- Andrade-Campos M., Alfonso P., Irun P., Armstrong J., Calvo C., Dalmau J., Domingo M.R., Barbera J.L., Cano H., Fernandez-Galán M.A., et al. Diagnosis features of pediatric Gaucher disease patients in the era of enzymatic therapy, a national-base study from the Spanish Registry of Gaucher disease. Orphanet. J. Rare Dis. 2017;12 doi: 10.1186/s13023-017-0627-z. - DOI - PMC - PubMed
-
- Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. Review of Gaucher disease pathophysiology, clinical presentation and treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

