Enhanced Enrichment of Medaka Ovarian Germline Stem Cells by a Combination of Density Gradient Centrifugation and Differential Plating
- PMID: 33114294
- PMCID: PMC7690863
- DOI: 10.3390/biom10111477
Enhanced Enrichment of Medaka Ovarian Germline Stem Cells by a Combination of Density Gradient Centrifugation and Differential Plating
Abstract
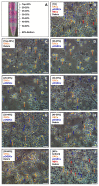
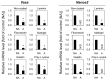
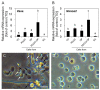
Fish ovarian germline stem cells (OGSCs) have great potential in various biological fields due to their ability to generate large numbers of mature eggs. Therefore, selective enrichment of OGSCs is a prerequisite for successful applications. To determine the optimal conditions for the enrichment of OGSCs from Japanese medaka (Oryzias latipes), we evaluated the effects of Percoll density gradient centrifugation (PDGC), differential plating (DP), and a combination of both methods. Based on cell morphology and gene expression of germ cell-specific Vasa and OGSC-specific Nanos2, we demonstrated that of seven density fractions obtained following PDGC, the 30-35% density fraction contained the highest proportion of OGSCs, and that Matrigel was the most effective biomolecule for the enrichment of Oryzias latipes OGSCs by DP in comparison to laminin, fibronectin, gelatin, and poly-l-lysine. Furthermore, we confirmed that PDGC and DP in combination significantly enhanced the efficiency of OGSC enrichment. The enriched cells were able to localize in the gonadal region at a higher efficiency compared to non-enriched ovarian cells when transplanted into the developing larvae. Our approach provides an efficient way to enrich OGSCs without using OGSC-specific surface markers or transgenic strains expressing OGSC-specific reporter proteins.
Keywords: Matrigel; density gradient centrifugation; differential plating; enrichment; fish; ovarian germline stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
[Toxic effect and mechanism of Tripterygium glycosides on ovarian germline stem cells of mice in vitro by Notch signaling pathway].Zhongguo Zhong Yao Za Zhi. 2024 Mar;49(6):1594-1601. doi: 10.19540/j.cnki.cjcmm.20231019.402. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 38621944 Chinese.
-
The Hippo Signaling Pathway Regulates Ovarian Function via the Proliferation of Ovarian Germline Stem Cells.Cell Physiol Biochem. 2017;41(3):1051-1062. doi: 10.1159/000464113. Epub 2017 Feb 27. Cell Physiol Biochem. 2017. PMID: 28245464
-
[Expression relationship of Hippo signaling molecules and ovarian germline stem cell markers in the ovarian aging process of women and mice].Sheng Li Xue Bao. 2019 Jun 25;71(3):405-414. Sheng Li Xue Bao. 2019. PMID: 31218331 Chinese.
-
Ovarian germline stem cells in the teleost fish, medaka (Oryzias latipes).Int J Biol Sci. 2011 Apr 14;7(4):403-9. doi: 10.7150/ijbs.7.403. Int J Biol Sci. 2011. PMID: 21547057 Free PMC article. Review.
-
Cross talk between germ cells and gonadal somatic cells is critical for sex differentiation of the gonads in the teleost fish, medaka (Oryzias latipes).Dev Growth Differ. 2008 May;50(4):273-8. doi: 10.1111/j.1440-169X.2008.01015.x. Dev Growth Differ. 2008. PMID: 18366386 Review.
Cited by
-
Advantages, Factors, Obstacles, Potential Solutions, and Recent Advances of Fish Germ Cell Transplantation for Aquaculture-A Practical Review.Animals (Basel). 2022 Feb 10;12(4):423. doi: 10.3390/ani12040423. Animals (Basel). 2022. PMID: 35203131 Free PMC article. Review.
-
The Primary Cultivation of Oogonial Stem Cells from Black Rockfish (Sebastes schlegelii): Morphology and Transcriptome Landscape.Int J Mol Sci. 2025 Jul 15;26(14):6772. doi: 10.3390/ijms26146772. Int J Mol Sci. 2025. PMID: 40725018 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

