Hijacking of Lipid Droplets by Hepatitis C, Dengue and Zika Viruses-From Viral Protein Moonlighting to Extracellular Release
- PMID: 33114346
- PMCID: PMC7662613
- DOI: 10.3390/ijms21217901
Hijacking of Lipid Droplets by Hepatitis C, Dengue and Zika Viruses-From Viral Protein Moonlighting to Extracellular Release
Abstract
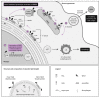
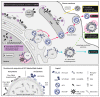
Hijacking and manipulation of host cell biosynthetic pathways by human enveloped viruses are essential for the viral lifecycle. Flaviviridae members, including hepatitis C, dengue and Zika viruses, extensively manipulate host lipid metabolism, underlining the importance of lipid droplets (LDs) in viral infection. LDs are dynamic cytoplasmic organelles that can act as sequestration platforms for a unique subset of host and viral proteins. Transient recruitment and mobilization of proteins to LDs during viral infection impacts host-cell biological properties, LD functionality and canonical protein functions. Notably, recent studies identified LDs in the nucleus and also identified that LDs are transported extracellularly via an autophagy-mediated mechanism, indicating a novel role for autophagy in Flaviviridae infections. These developments underline an unsuspected diversity and localization of LDs and potential moonlighting functions of LD-associated proteins during infection. This review summarizes recent breakthroughs concerning the LD hijacking activities of hepatitis C, dengue and Zika viruses and potential roles of cytoplasmic, nuclear and extracellular LD-associated viral proteins during infection.
Keywords: SREBP (sterol regulatory element-binding protein) pathway; Zika virus; autophagy; dengue virus; hepatitis C virus; lipid droplets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

