Implementing ctDNA Analysis in the Clinic: Challenges and Opportunities in Non-Small Cell Lung Cancer
- PMID: 33114393
- PMCID: PMC7693855
- DOI: 10.3390/cancers12113112
Implementing ctDNA Analysis in the Clinic: Challenges and Opportunities in Non-Small Cell Lung Cancer
Abstract
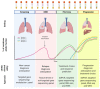
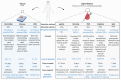
Tumor genomic profiling has a dramatic impact on the selection of targeted treatment and for the identification of resistance mechanisms at the time of progression. Solid tissue biopsies are sometimes challenging, and liquid biopsies are used as a non-invasive alternative when tissue is limiting. The clinical relevance of tumor genotyping through analysis of ctDNA is now widely recognized at all steps of the clinical evaluation process in metastatic non-small cell lung cancer (NSCLC) patients. ctDNA analysis through liquid biopsy has recently gained increasing attention as well in the management of early and locally advanced, not oncogene-addicted, NSCLC. Its potential applications in early disease detection and the response evaluation to radical treatments are promising. The aim of this review is to summarize the landscape of liquid biopsies in clinical practice and also to provide an overview of the potential perspectives of development focusing on early detection and screening, the assessment of minimal residual disease, and its potential role in predicting response to immunotherapy. In addition to available studies demonstrating the clinical relevance of liquid biopsies, there is a need for standardization and well-designed clinical trials to demonstrate its clinical utility.
Keywords: biomarker; circulating tumor DNA; liquid biopsy; lung cancer; next-generation sequencing.
Conflict of interest statement
P.S. received research funding from Illumina, Inivata, Roche, AstraZeneca, Bristol-Myers-Squibb, and HTG Diagnostics. E.G. received personal fees from AstraZeneca, Roche, Merck Sharp and Dohme, and Bristol-Myers Squibb, as well as a research grant from Bristol-Myers Squibb outside the submitted work. A.S. received personal fees from Roche, Bristol-Myers Squibb, Takeda, Lilly, Pfizer, AstraZeneca, and Boehringer-Ingelheim. D.M.S. received personal fees from Roche, Merck Sharpe and Dohme, Bristol-Myers Squibb, and AstraZeneca outside the submitted work. ACT received personal fees from AstraZeneca, Roche, Merck Sharp and Dohme, and Bristol-Myers Squibb, outside the submitted work. M.G.L. received personal fees from AstraZeneca, Roche, Merck Sharp and Dohme, and Bristol-Myers Squibb, outside the submitted work. MP received personal fees from Illumina and fundings from Inivata for an institutional project. S.O.C. had no conflict of interest.
Figures
References
-
- Rolfo C., Mack P.C., Scagliotti G.V., Baas P., Barlesi F., Bivona T., Herbst R.S., Mok T.S., Peled N., Pirker R., et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018;13:1248–1268. doi: 10.1016/j.jtho.2018.05.030. - DOI - PubMed
-
- Blakely C.M., Wu W., Gubens M.A., Rotow J., Wang V., Banks K.C., Lanman R.B., Mack P.C., Husain H., McCoach C.E., et al. Evolution and clinical impact of genomic alterations detectable in circulating tumor DNA of 1150 advanced EGFR-mutant (mt) lung cancer patients. J. Clin. Oncol. 2017;35:9009. doi: 10.1200/JCO.2017.35.15_suppl.9009. - DOI
-
- McCoach C.E., Blakely C.M., Banks K.C., Levy B.M., Chue B.M., Raymond V.M., Le A.T., Lee C.E., Diaz J., Waqar S.N., et al. Clinical Utility of Cell-Free DNA for the Detection ofALKFusions and Genomic Mechanisms of ALK Inhibitor Resistance in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2018;24:2758–2770. doi: 10.1158/1078-0432.CCR-17-2588. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical