hATTR Pathology: Nerve Biopsy Results from Italian Referral Centers
- PMID: 33114611
- PMCID: PMC7692609
- DOI: 10.3390/brainsci10110780
hATTR Pathology: Nerve Biopsy Results from Italian Referral Centers
Abstract
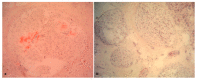
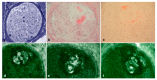
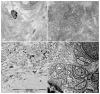
Pathological evidence of amyloid on nerve biopsy has been the gold standard for diagnosis in hereditary transthyretin amyloidosis polyneuropathy (hATTR-PN) for a long time. In this article, we reviewed the pathological findings of a large series of sural nerve biopsies from a cohort of hATTR-PN patients, collected by different Italian referral centers. Patients and Methods: We reviewed clinical and pathological data from hATTR-PN patients, diagnosed and followed in five Italian referral centers for peripheral neuropathies. Diagnosis was formulated after a positive genetic test for transthyretin (TTR) mutations. Sural nerve biopsy was performed according to standard protocols. Results: Sixty-nine sural nerve biopsies from hATTR-PN patients were examined. Congo red positive deposits were found in 73% of cases. Only the Phe64Leu mutation failed to show amyloid deposits in a high percentage of biopsies (54%), as already described. Unusual pathological findings, such as myelin abnormalities or inflammatory infiltrates, were detected in occasional cases. Conclusions: Even if no longer indicated to confirm hATTR-PN clinical suspicion, nerve biopsy remains, in expert hands, a rapid and inexpensive tool to detect amyloid deposition. In Italy, clinicians should be aware that a negative biopsy does not exclude hATTR-PN, particularly for Phe64Leu, one of the most frequent mutations in this country.
Keywords: Congo red; amyloid; axonal loss; hATTR; nerve biopsy; polyneuropathy.
Conflict of interest statement
M.L. received financial grants (honoraria and speaking) from Ackea, Alnylam, and Pfizer, and travel grants from Ackea, Alnylam, Pfizer, Kedrion, Csl Behring, and Grifols; G.B. received financial grants (honoraria and speaking) from Alnylam and travel grants from Pfizer, Alnylam, and Grifols; A.D.P. received travel grants from Pfizer; G.M.F. acknowledges donations from Akcea and Pfizer to support activities of his research unit, financial support from Pfizer, Kedrion, and Akcea for participation in national and international meetings, and participation in the advisory boards of Vitacess, Alnylam, and Akcea; speaker honorarium from Akcea; S.F. received financial grants (honoraria and speaking) from Alnylam, Akcea, and Pfizer and travel grants from Alnylam and Akcea; A.M. discloses having been on the advisory board for Alnylam Therap., Akcea Therap., and Pfizer, and she is also the principal investigator in clinical trials sponsored by Alnylam Therap.; D.P. received financial grants (honoraria and speaking) from Alnylam, Akcea, Pfizer, and Inflectis, and travel grants from Kedrion and Pfizer; A.R. received travel grants from Pfizer and Csl Behring, and a financial grant from Akcea; M.S. received financial grants (honoraria and speaking) from Ackea and Alnylam and travel grants from Grifols. Other authors have no potential conflicts of interest to be disclosed. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Luigetti M., Conte A., Del Grande A., Bisogni G., Madia F., Lo Monaco M., Laurenti L., Obici L., Merlini G., Sabatelli M. TTR-related amyloid neuropathy: Clinical, electrophysiological and pathological findings in 15 unrelated patients. Neurol. Sci. 2013;34:1057–1063. doi: 10.1007/s10072-012-1105-y. - DOI - PubMed
-
- Russo M., Mazzeo A., Stancanelli C., Di Leo R., Gentile L., Di Bella G., Minutoli F., Baldari S., Vita G. Transthyretin-related familial amyloidotic polyneuropathy: Description of a cohort of patients with Leu64 mutation and late onset. J. Peripher. Nerv. Syst. 2012;17:385–390. doi: 10.1111/j.1529-8027.2012.00436.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

