The Basal Pharmacology of Palmitoylethanolamide
- PMID: 33114698
- PMCID: PMC7662788
- DOI: 10.3390/ijms21217942
The Basal Pharmacology of Palmitoylethanolamide
Abstract
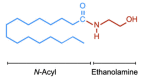
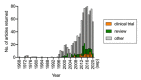
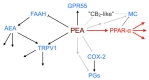
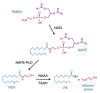
Palmitoylethanolamide (PEA, N-hexadecanoylethanolamide) is an endogenous compound belonging to the family of N-acylethanolamines. PEA has anti-inflammatory and analgesic properties and is very well tolerated in humans. In the present article, the basal pharmacology of PEA is reviewed. In terms of its pharmacokinetic properties, most work has been undertaken upon designing formulations for its absorption and upon characterising the enzymes involved in its metabolism, but little is known about its bioavailability, tissue distribution, and excretion pathways. PEA exerts most of its biological effects in the body secondary to the activation of peroxisome proliferator-activated receptor-α (PPAR-α), but PPAR-α-independent pathways involving other receptors (Transient Receptor Potential Vanilloid 1 (TRPV1), GPR55) have also been identified. Given the potential clinical utility of PEA, not least for the treatment of pain where there is a clear need for new well-tolerated drugs, we conclude that the gaps in our knowledge, in particular those relating to the pharmacokinetic properties of the compound, need to be filled.
Keywords: N-acylethanolamine acid amidase; atopic eczema; fatty acid amide hydrolase; low back pain–sciatica; palmitoylethanolamide; peroxisome proliferator-activated receptor-α.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kuehl F., Jacob T., Ganley O., Ormond R., Meisinger M. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 1957;79:5577–5578. doi: 10.1021/ja01577a066. - DOI
-
- Bachur N., Masek K., Melmon K., Udenfriend S. Fatty acid amides of ethanolamine in mammalian tissues. J. Biol. Chem. 1965;240:1019–1024. - PubMed
-
- Coburn A., Moore L. Nutrition as a conditioning factor in the rheumatic state. Am. J. Dis. Child. 1943;65:744–756. doi: 10.1001/archpedi.1943.02010170066008. - DOI
-
- Guida G., De Martino M., De Fabiani A., Cantieri L.A., Alexandre A., Vassallo G.M., Rogai M., Lanaia F., Petrosino S. La palmitoilethanolamida (Normast) en el dolor neuropático crónico por lumbociatalgia de tipo compresivo: Estudio clínico multicéntrico. Dolor. 2010;25:35–42.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

