Natural Inspired Carboxymethyl Cellulose (CMC) Doped with Ammonium Carbonate (AC) as Biopolymer Electrolyte
- PMID: 33114745
- PMCID: PMC7693293
- DOI: 10.3390/polym12112487
Natural Inspired Carboxymethyl Cellulose (CMC) Doped with Ammonium Carbonate (AC) as Biopolymer Electrolyte
Abstract
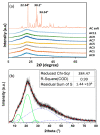
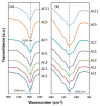
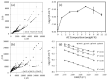
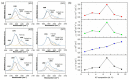
Green and safer materials in energy storage technology are important right now due to increased consumption. In this study, a biopolymer electrolyte inspired from natural materials was developed by using carboxymethyl cellulose (CMC) as the core material and doped with varied ammonium carbonate (AC) composition. X-ray diffraction (XRD) shows the prepared CMC-AC electrolyte films exhibited low crystallinity content, Xc (~30%) for sample AC7. A specific wavenumber range between 900-1200 cm-1 and 1500-1800 cm-1 was emphasized in Fourier transform infrared (FTIR) testing, as this is the most probable interaction to occur. The highest ionic conductivity, σ of the electrolyte system achieved was 7.71 × 10-6 Scm-1 and appeared greatly dependent on ionic mobility, µ and diffusion coefficient, D. The number of mobile ions, η, increased up to the highest conducting sample (AC7) but it became less prominent at higher AC composition. The transference measurement, tion showed that the electrolyte system was predominantly ionic with sample AC7 having the highest value (tion = 0.98). Further assessment also proved that the H+ ion was the main conducting species in the CMC-AC electrolyte system, which presumably was due to protonation of ammonium salt onto the complexes site and contributed to the overall ionic conductivity enhancement.
Keywords: biopolymer; carboxymethyl cellulose; ionic transport; solid polymer electrolyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shi S., Gao J., Liu Y., Zhao Y., Wu Q., Ju W., Ouyang C., Xiao R. Multi-scale computation methods: Their applications in lithium-ion battery research and development. Chin. Phys. B. 2016;25:018212. doi: 10.1088/1674-1056/25/1/018212. - DOI
-
- Goodenough J.B., Kim Y.S. Challenges for rechargeable batteries. J. Power Sources. 2011;196:6688–6694. doi: 10.1016/j.jpowsour.2010.11.074. - DOI
-
- Kerdphol T., Qudaih Y., Mitani Y. Optimum battery energy storage system using PSO considering dynamic demand response for microgrids. Int. J. Electr. Power Energy Syst. 2016;83:58–66. doi: 10.1016/j.ijepes.2016.03.064. - DOI
-
- Yue L.P., Ma J., Zhang J.J., Zhao J.W., Dong S.M., Liu Z.H., Cui G.L., Chen L.Q. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016;5:139–164. doi: 10.1016/j.ensm.2016.07.003. - DOI
-
- Chai M.N., Isa M.I.N. Electrical Characterization and Ionic Transport Properties of Carboxyl Methylcellulose-Oleic Acid Solid Polymer Electrolytes. Int. J. Polym. Anal. Charact. 2013;18:280–286. doi: 10.1080/1023666X.2013.767033. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

