Nonalcoholic Fatty Liver Disease: A Drug Revolution Is Coming
- PMID: 33115209
- PMCID: PMC7643594
- DOI: 10.4093/dmj.2020.0115
Nonalcoholic Fatty Liver Disease: A Drug Revolution Is Coming
Abstract
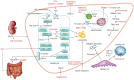
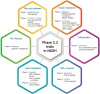
The worldwide prevalence of non-alcoholic fatty liver disease is around 25%, and that of nonalcoholic steatohepatitis (NASH) ranges from 1.5% to 6.45%. Patients with NASH, especially those with fibrosis, are at higher risk for adverse outcomes such as cirrhosis and liver-related mortality. Although vitamin E, pioglitazone, and liraglutide improved liver histology in randomized trials, there are currently no Food and Drug Administration-approved drugs for NASH. Five pharmacologic agents-obeticholic acid, elafibranor, cenicriviroc, resmetirom, and aramchol-are being evaluated in large, histology-based phase 3 trials. Within 2 to 4 years, new and effective drugs for the treatment of NASH are expected. Additionally, many phase 2 trials are ongoing for various agents. Based on the results of phase 2 and 3 trials, combination treatments are also being investigated. Future treatment strategies will comprise drug combinations and precision medicine based on the different phenotypes of NASH and treatment response of the individual patient.
Keywords: Fibrosis; Non-alcoholic fatty liver disease; Precision medicine; Therapeutics; Drug therapy, combination.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. - PMC - PubMed
-
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. - PubMed
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. - PubMed
-
- Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

