Hypoxia Induces Transcriptional and Translational Downregulation of the Type I IFN Pathway in Multiple Cancer Cell Types
- PMID: 33115807
- PMCID: PMC7611234
- DOI: 10.1158/0008-5472.CAN-19-2306
Hypoxia Induces Transcriptional and Translational Downregulation of the Type I IFN Pathway in Multiple Cancer Cell Types
Abstract
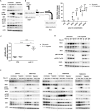
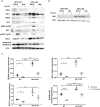
Hypoxia is a common phenomenon in solid tumors and is strongly linked to hallmarks of cancer. Recent evidence has shown that hypoxia promotes local immune suppression. Type I IFN supports cytotoxic T lymphocytes by stimulating the maturation of dendritic cells and enhancing their capacity to process and present antigens. However, little is known about the relationship between hypoxia and the type I IFN pathway, which comprises the sensing of double-stranded RNA and DNA (dsRNA/dsDNA) followed by IFNα/β secretion and transcriptional activation of IFN-stimulated genes (ISG). In this study, we determined the effects of hypoxia on the type I IFN pathway in breast cancer and the mechanisms involved. In cancer cell lines and xenograft models, mRNA and protein expressions of the type I IFN pathway were downregulated under hypoxic conditions. This pathway was suppressed at each level of signaling, from the dsRNA sensors RIG-I and MDA5, the adaptor MAVS, transcription factors IRF3, IRF7, and STAT1, and several ISG including RIG-I, IRF7, STAT1, and ADAR-p150. Importantly, IFN secretion was reduced under hypoxic conditions. HIF1α- and HIF2α-mediated regulation of gene expression did not explain most of the effects. However, ATAC-seq data revealed in hypoxia that peaks with STAT1 and IRF3 motifs had decreased accessibility. Collectively, these results indicate that hypoxia leads to an overall downregulation of the type I IFN pathway due to repressed transcription and lower chromatin accessibility in an HIF1/2α-independent manner, which could contribute to immunosuppression in hypoxic tumors. SIGNIFICANCE: These findings characterize a new mechanism of immunosuppression by hypoxia via downregulation of the type I IFN pathway and its autocrine/paracrine effects on tumor growth.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures





References
-
- Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009;107:1053–62. - PubMed
-
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. - PubMed
-
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

