A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease
- PMID: 33115952
- PMCID: PMC7926267
- DOI: 10.1126/scitranslmed.abb7656
A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease
Abstract
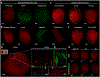
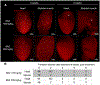
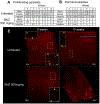
A major contributor to treatment failure in Chagas disease, caused by infection with the protozoan parasite Trypanosoma cruzi, is that current treatment regimens do not address the drug insensitivity of transiently dormant T. cruzi amastigotes. Here, we demonstrated that use of a currently available drug in a modified treatment regimen of higher individual doses, given less frequently over an extended treatment period, could consistently extinguish T. cruzi infection in three mouse models of Chagas disease. Once per week administration of benznidazole at a dose 2.5 to 5 times the standard daily dose rapidly eliminated actively replicating parasites and ultimately eradicated the residual, transiently dormant parasite population in mice. This outcome was initially confirmed in "difficult to cure" mouse infection models using immunological, parasitological, and molecular biological approaches and ultimately corroborated by whole organ analysis of optically clarified tissues using light sheet fluorescence microscopy (LSFM). This tool was effective for monitoring pathogen load in intact organs, including detection of individual dormant parasites, and for assessing treatment outcomes. LSFM-based analysis also suggested that dormant amastigotes of T. cruzi may not be fully resistant to trypanocidal compounds such as benznidazole. Collectively, these studies provide important information on the phenomenon of dormancy in T. cruzi infection in mice, demonstrate methods to therapeutically override dormancy using a currently available drug, and provide methods to monitor alternative therapeutic approaches for this, and possibly other, low-density infectious agents.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Schofield CJ, Jannin J, Salvatella R, The future of Chagas disease control. Trends Parasitol 22, 583–588 (2006). - PubMed
-
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L, Control of neglected tropical diseases. N Engl J Med 357, 1018–1027 (2007). - PubMed
-
- WHO, Chagas disease in Latin America: an epidemiological update based on 2010 estimates. WHO Weekly Epidemiological Record (WER) 90, 33–44 (2015). - PubMed
-
- Rodriques Coura J, de Castro SL, A critical review on Chagas disease chemotherapy. Memorias do Instituto Oswaldo Cruz 97, 3–24 (2002). - PubMed
-
- Urbina JA, Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta tropica 115, 55–68 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

