Mohawk is a transcription factor that promotes meniscus cell phenotype and tissue repair and reduces osteoarthritis severity
- PMID: 33115953
- PMCID: PMC7955769
- DOI: 10.1126/scitranslmed.aan7967
Mohawk is a transcription factor that promotes meniscus cell phenotype and tissue repair and reduces osteoarthritis severity
Abstract
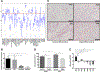
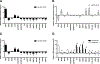
Meniscus tears are common knee injuries and a major osteoarthritis (OA) risk factor. Knowledge gaps that limit the development of therapies for meniscus injury and degeneration concern transcription factors that control the meniscus cell phenotype. Analysis of RNA sequencing data from 37 human tissues in the Genotype-Tissue Expression database and RNA sequencing data from meniscus and articular cartilage showed that transcription factor Mohawk (MKX) is highly enriched in meniscus. In human meniscus cells, MKX regulates the expression of meniscus marker genes, OA-related genes, and other transcription factors, including Scleraxis (SCX), SRY Box 5 (SOX5), and Runt domain-related transcription factor 2 (RUNX2). In mesenchymal stem cells (MSCs), the combination of adenoviral MKX (Ad-MKX) and transforming growth factor-β3 (TGF-β3) induced a meniscus cell phenotype. When Ad-MKX-transduced MSCs were seeded on TGF-β3-conjugated decellularized meniscus scaffold (DMS) and inserted into experimental tears in meniscus explants, they increased glycosaminoglycan content, extracellular matrix interconnectivity, cell infiltration into the DMS, and improved biomechanical properties. Ad-MKX injection into mouse knee joints with experimental OA induced by surgical destabilization of the meniscus suppressed meniscus and cartilage damage, reducing OA severity. Ad-MKX injection into human OA meniscus tissue explants corrected pathogenic gene expression. These results identify MKX as a previously unidentified key transcription factor that regulates the meniscus cell phenotype. The combination of Ad-MKX with TGF-β3 is effective for differentiation of MSCs to a meniscus cell phenotype and useful for meniscus repair. MKX is a promising therapeutic target for meniscus tissue engineering, repair, and prevention of OA.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
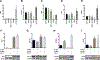




References
-
- Laible C, Stein DA, Kiridly DN, Meniscal repair. J Am Acad Orthop Surg 21, 204–213 (2013). - PubMed
-
- DeHaven KE, Meniscus repair. Am J Sports Med 27, 242–250 (1999). - PubMed
-
- Keene GC, Bickerstaff D, Rae PJ, Paterson RS, The natural history of meniscal tears in anterior cruciate ligament insufficiency. Am J Sports Med 21, 672–679 (1993). - PubMed
-
- Cipolla M, Scala A, Gianni E, Puddu G, Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Classification, staging and timing of treatment. Knee Surg Sports Traumatol Arthrosc 3, 130–134 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

