The silk of gorse spider mite Tetranychus lintearius represents a novel natural source of nanoparticles and biomaterials
- PMID: 33116211
- PMCID: PMC7595037
- DOI: 10.1038/s41598-020-74766-7
The silk of gorse spider mite Tetranychus lintearius represents a novel natural source of nanoparticles and biomaterials
Abstract
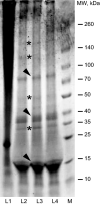
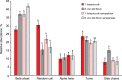
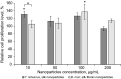
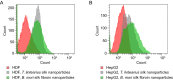
Spider mites constitute an assemblage of well-known pests in agriculture, but are less known for their ability to spin silk of nanoscale diameters and high Young's moduli. Here, we characterize silk of the gorse spider mite Tetranychus lintearius, which produces copious amounts of silk with nano-dimensions. We determined biophysical characteristics of the silk fibres and manufactured nanoparticles and biofilm derived from native silk. We determined silk structure using attenuated total reflectance Fourier transform infrared spectroscopy, and characterized silk nanoparticles using field emission scanning electron microscopy. Comparative studies using T. lintearius and silkworm silk nanoparticles and biofilm demonstrated that spider mite silk supports mammalian cell growth in vitro and that fluorescently labelled nanoparticles can enter cell cytoplasm. The potential for cytocompatibility demonstrated by this study, together with the prospect of recombinant silk production, opens a new avenue for biomedical application of this little-known silk.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Proteomic evidence for the silk fibroin genes of spider mites (Order Trombidiformes: Family Tetranychidae).J Proteomics. 2021 May 15;239:104195. doi: 10.1016/j.jprot.2021.104195. Epub 2021 Mar 20. J Proteomics. 2021. PMID: 33757880
-
How to visualize the spider mite silk?Microsc Res Tech. 2009 Sep;72(9):659-64. doi: 10.1002/jemt.20712. Microsc Res Tech. 2009. PMID: 19322898
-
Using mathematical models to describe aerial dispersal and silk ball formation of peanut red spider mite, Tetranychus ogmophallos (Acari: Tetranychidae).Exp Appl Acarol. 2020 May;81(1):85-102. doi: 10.1007/s10493-020-00495-1. Epub 2020 Apr 28. Exp Appl Acarol. 2020. PMID: 32347429
-
Ancient fibrous biomaterials from silkworm protein fibroin and spider silk blends: Biomechanical patterns.Acta Biomater. 2022 Nov;153:38-67. doi: 10.1016/j.actbio.2022.09.030. Epub 2022 Sep 17. Acta Biomater. 2022. PMID: 36126911 Review.
-
Biotechnological production of spider-silk proteins enables new applications.Macromol Biosci. 2007 Apr 10;7(4):401-9. doi: 10.1002/mabi.200600255. Macromol Biosci. 2007. PMID: 17429812 Review.
Cited by
-
Smart nanomaterial and nanocomposite with advanced agrochemical activities.Nanoscale Res Lett. 2021 Oct 18;16(1):156. doi: 10.1186/s11671-021-03612-0. Nanoscale Res Lett. 2021. PMID: 34664133 Free PMC article. Review.
-
Preparation and Characterization of Photo-Cross-Linkable Methacrylated Silk Fibroin and Methacrylated Hyaluronic Acid Composite Hydrogels.Biomacromolecules. 2024 Nov 11;25(11):7078-7097. doi: 10.1021/acs.biomac.4c00319. Epub 2024 Oct 14. Biomacromolecules. 2024. PMID: 39401165 Free PMC article.
-
Microscopic Menaces: The Impact of Mites on Human Health.Int J Mol Sci. 2024 Mar 26;25(7):3675. doi: 10.3390/ijms25073675. Int J Mol Sci. 2024. PMID: 38612486 Free PMC article. Review.
-
Identification of the fibroin of Stigmaeopsis nanjingensis by a nanocarrier-based transdermal dsRNA delivery system.Exp Appl Acarol. 2022 May;87(1):31-47. doi: 10.1007/s10493-022-00718-7. Epub 2022 May 11. Exp Appl Acarol. 2022. PMID: 35543822 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

