Parallel ascending spinal pathways for affective touch and pain
- PMID: 33116307
- PMCID: PMC7666110
- DOI: 10.1038/s41586-020-2860-1
Parallel ascending spinal pathways for affective touch and pain
Abstract
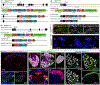
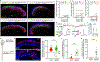
The anterolateral pathway consists of ascending spinal tracts that convey pain, temperature and touch information from the spinal cord to the brain1-4. Projection neurons of the anterolateral pathway are attractive therapeutic targets for pain treatment because nociceptive signals emanating from the periphery are channelled through these spinal projection neurons en route to the brain. However, the organizational logic of the anterolateral pathway remains poorly understood. Here we show that two populations of projection neurons that express the structurally related G-protein-coupled receptors (GPCRs) TACR1 and GPR83 form parallel ascending circuit modules that cooperate to convey thermal, tactile and noxious cutaneous signals from the spinal cord to the lateral parabrachial nucleus of the pons. Within this nucleus, axons of spinoparabrachial (SPB) neurons that express Tacr1 or Gpr83 innervate distinct sets of subnuclei, and strong optogenetic stimulation of the axon terminals induces distinct escape behaviours and autonomic responses. Moreover, SPB neurons that express Gpr83 are highly sensitive to cutaneous mechanical stimuli and receive strong synaptic inputs from both high- and low-threshold primary mechanosensory neurons. Notably, the valence associated with activation of SPB neurons that express Gpr83 can be either positive or negative, depending on stimulus intensity. These findings reveal anatomically, physiologically and functionally distinct subdivisions of the SPB tract that underlie affective aspects of touch and pain.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


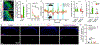


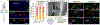






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

